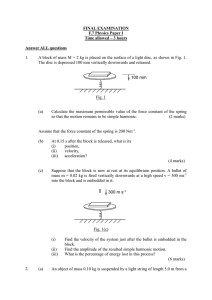
H - Quantum Condensed Matter Research Group
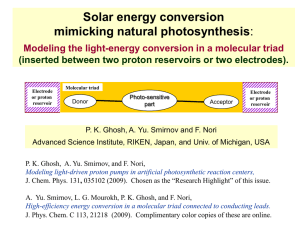
... J. Phys. Chem. C 113, 21218 (2009). Complimentary color copies of these are online. ...
... J. Phys. Chem. C 113, 21218 (2009). Complimentary color copies of these are online. ...
Transition form factor of the hydrogen Rydberg atom
... where u C i & and u C f & are the electron wave functions for the initial and final atomic states, respectively, and p is the momentum transferred to electrons ~atomic units are used throughout the paper!. The square of Eq. ~1.1!, u T f i u 2 , is the transition probability from the state i to the s ...
... where u C i & and u C f & are the electron wave functions for the initial and final atomic states, respectively, and p is the momentum transferred to electrons ~atomic units are used throughout the paper!. The square of Eq. ~1.1!, u T f i u 2 , is the transition probability from the state i to the s ...
Lecture notes
... This book appears every two years in two versions: the book and the booklet. Both of them list all aspects of the known particles and forces. The book also contains concise, but excellent short reviews of theories, experiments, accellerators, analysis techniques, statistics etc. There is also a vers ...
... This book appears every two years in two versions: the book and the booklet. Both of them list all aspects of the known particles and forces. The book also contains concise, but excellent short reviews of theories, experiments, accellerators, analysis techniques, statistics etc. There is also a vers ...
Review Study Guide for the Final
... What is it called when you have more electrons than protons? ...
... What is it called when you have more electrons than protons? ...
the hydrogen atom in a uniform magnetic field - an example
... since the beginning of this century. However in recent years it has become increasingly clear that even seemingly simple systems with few degrees of freedom generally show chaotic behaviour, and advances in computer technology have made it possible to study irregular motion in small systems in consi ...
... since the beginning of this century. However in recent years it has become increasingly clear that even seemingly simple systems with few degrees of freedom generally show chaotic behaviour, and advances in computer technology have made it possible to study irregular motion in small systems in consi ...
Chemistry 133 Problem Set Introduction
... rim with water and re-weighed, the mass is 55.78 g. A small piece of metal is then gently dropped into the filled beaker, causing a total of 1.55 g of water to overflow. The total mass of the beaker, the remaining water, and the metal is 68.02 g. The density of water is 1.00 g/mL. Determine the dens ...
... rim with water and re-weighed, the mass is 55.78 g. A small piece of metal is then gently dropped into the filled beaker, causing a total of 1.55 g of water to overflow. The total mass of the beaker, the remaining water, and the metal is 68.02 g. The density of water is 1.00 g/mL. Determine the dens ...
A – Momentum - cloudfront.net
... 1. What is the momentum of a 3000 kg truck traveling at 25 m/s? 2. A 1500 kg ferryboat has a momentum of 25000 kg∙m/s. What is the speed of the ferryboat? 3. A car travels at a constant speed of 24 m/s and has a momentum of 28800 kg∙m/s. What is the mass of the car? 4. An 8 kg bowling ball rolls at ...
... 1. What is the momentum of a 3000 kg truck traveling at 25 m/s? 2. A 1500 kg ferryboat has a momentum of 25000 kg∙m/s. What is the speed of the ferryboat? 3. A car travels at a constant speed of 24 m/s and has a momentum of 28800 kg∙m/s. What is the mass of the car? 4. An 8 kg bowling ball rolls at ...
Questions - Scheikundeolympiade
... The muon () is a subatomic particle of the lepton family which has same charge and magnetic behavior as the electron, but has a different mass and is unstable, i.e., it disintegrates into other particles within microseconds after its creation. Here you will attempt to determine the mass of the muon ...
... The muon () is a subatomic particle of the lepton family which has same charge and magnetic behavior as the electron, but has a different mass and is unstable, i.e., it disintegrates into other particles within microseconds after its creation. Here you will attempt to determine the mass of the muon ...
Rotational Motion
... 5. Repeat step 3 & 4 at least four more times. 6. Repeat steps 1-5 at least three more times with different amounts of weights. (You should have a whole box of weights to choose from; you decide how much weight you want to add.) Part III: Changing the Applied Torque by Changing the Radius Unfortunat ...
... 5. Repeat step 3 & 4 at least four more times. 6. Repeat steps 1-5 at least three more times with different amounts of weights. (You should have a whole box of weights to choose from; you decide how much weight you want to add.) Part III: Changing the Applied Torque by Changing the Radius Unfortunat ...
Parton model from bi-local solitonic picture of the baryon in two-dimensions
... we have a reconciliation of the exact bi-local soliton model with the simpler relativistic parton picture of the baryon, in 2 dimensions. The main advantage of this new point of view is that the semi-classical approximation of hadron dynamics corresponds to the large Nc limit of 2d QCD, and so is ca ...
... we have a reconciliation of the exact bi-local soliton model with the simpler relativistic parton picture of the baryon, in 2 dimensions. The main advantage of this new point of view is that the semi-classical approximation of hadron dynamics corresponds to the large Nc limit of 2d QCD, and so is ca ...
Chapter 21 Rigid Body Dynamics: Rotation and Translation
... a reference frame moving with the center of mass, we can analyze the rotational motion separately and discover that the torque about the center of mass is equal to the change in the angular momentum about the center of mass. For a rigid body undergoing fixed axis rotation about the center of mass, o ...
... a reference frame moving with the center of mass, we can analyze the rotational motion separately and discover that the torque about the center of mass is equal to the change in the angular momentum about the center of mass. For a rigid body undergoing fixed axis rotation about the center of mass, o ...
Chapter 7 Lecture
... • Complete ionic equations show aqueous ionic compounds that normally dissociate in solution as they are actually present in solution. • When writing complete ionic equations, separate only aqueous ionic compounds into their constituent ions. • Do NOT separate solid, liquid, or gaseous compounds. ...
... • Complete ionic equations show aqueous ionic compounds that normally dissociate in solution as they are actually present in solution. • When writing complete ionic equations, separate only aqueous ionic compounds into their constituent ions. • Do NOT separate solid, liquid, or gaseous compounds. ...
Vortex states of a disordered quantum Hall bilayer P. R. Eastham,
... modulation-doped samples. We argue that for a fixed disorder potential there is a characteristic value of the magnetic length, above which vortices proliferate. We find that this proliferation corresponds to the formation of an emulsion of vortex-antivortex crystals. Our theory should be testable si ...
... modulation-doped samples. We argue that for a fixed disorder potential there is a characteristic value of the magnetic length, above which vortices proliferate. We find that this proliferation corresponds to the formation of an emulsion of vortex-antivortex crystals. Our theory should be testable si ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.