Experimental Techniques in Nuclear
... However, physics is an experimental science and physics students should understand how experiments work, and be able to make experiments work. As a post doc, I have never designed any electronics board, but many times I have had to find out why the board I have given did not do what it was supposed ...
... However, physics is an experimental science and physics students should understand how experiments work, and be able to make experiments work. As a post doc, I have never designed any electronics board, but many times I have had to find out why the board I have given did not do what it was supposed ...
Physics v. 2016
... and gravitation to describe and predict the motion of objects ranging from atoms to the galaxies. 3.2.12.B6. CONSTANCY/CHANGE Compare and contrast motions of objects using forces and conservation laws ...
... and gravitation to describe and predict the motion of objects ranging from atoms to the galaxies. 3.2.12.B6. CONSTANCY/CHANGE Compare and contrast motions of objects using forces and conservation laws ...
Document
... That means we can measure the distance between two particles and their total momentum, to arbitrary precision. EPR claimed that we can measure x2 or p2 without affecting Particle 2 in any way, via Particle 1 (and vice versa). If so, then x2 and p2 have simultaneous values. Quantum mechanics has no p ...
... That means we can measure the distance between two particles and their total momentum, to arbitrary precision. EPR claimed that we can measure x2 or p2 without affecting Particle 2 in any way, via Particle 1 (and vice versa). If so, then x2 and p2 have simultaneous values. Quantum mechanics has no p ...
An experimental and theoretical guide to strongly interacting
... the implementation of various quantum simulation and quantum computation protocols but also for other tasks involving interacting Rydberg atoms. One could be the idea of combining interacting Rydberg atoms with quantum degenerate gases [47–50] to render new interaction mechanisms. Besides the effect ...
... the implementation of various quantum simulation and quantum computation protocols but also for other tasks involving interacting Rydberg atoms. One could be the idea of combining interacting Rydberg atoms with quantum degenerate gases [47–50] to render new interaction mechanisms. Besides the effect ...
FEATURE ARTICLE
... interest in these techniques,53,54 and also their applicability in an ab initio context is being investigated.55,56 Although the high degree of reliability of the DFT calculations based on the present functionals, usually referred to as generalized gradient approximations (GGA), and their computatio ...
... interest in these techniques,53,54 and also their applicability in an ab initio context is being investigated.55,56 Although the high degree of reliability of the DFT calculations based on the present functionals, usually referred to as generalized gradient approximations (GGA), and their computatio ...
PowerPoint Lecture Slides: Chap. 3
... 2. Convert the known mass of the reactant or product to moles of that substance. 3. Use the balanced equation to set up the appropriate mole ratios. 4. Use the appropriate mole ratios to calculate the number of moles of desired reactant or product. 5. Convert from moles back to grams if required by ...
... 2. Convert the known mass of the reactant or product to moles of that substance. 3. Use the balanced equation to set up the appropriate mole ratios. 4. Use the appropriate mole ratios to calculate the number of moles of desired reactant or product. 5. Convert from moles back to grams if required by ...
Chapter 3
... 2. Convert the known mass of the reactant or product to moles of that substance. 3. Use the balanced equation to set up the appropriate mole ratios. 4. Use the appropriate mole ratios to calculate the number of moles of desired reactant or product. 5. Convert from moles back to grams if required by ...
... 2. Convert the known mass of the reactant or product to moles of that substance. 3. Use the balanced equation to set up the appropriate mole ratios. 4. Use the appropriate mole ratios to calculate the number of moles of desired reactant or product. 5. Convert from moles back to grams if required by ...
Chemistry 6–12
... (e.g., melting point, boiling point, vapor pressure, solubility, conductivity) of matter. Solve problems involving an intensive property (e.g., density, specific heat) of matter. Differentiate between various physical methods (e.g., chromatography, distillation, filtration) for separating the compon ...
... (e.g., melting point, boiling point, vapor pressure, solubility, conductivity) of matter. Solve problems involving an intensive property (e.g., density, specific heat) of matter. Differentiate between various physical methods (e.g., chromatography, distillation, filtration) for separating the compon ...
Ch. 3 PP - Lemon Bay High School
... 2. Convert the known mass of the reactant or product to moles of that substance. 3. Use the balanced equation to set up the appropriate mole ratios. 4. Use the appropriate mole ratios to calculate the number of moles of desired reactant or product. 5. Convert from moles back to grams if required by ...
... 2. Convert the known mass of the reactant or product to moles of that substance. 3. Use the balanced equation to set up the appropriate mole ratios. 4. Use the appropriate mole ratios to calculate the number of moles of desired reactant or product. 5. Convert from moles back to grams if required by ...
AQA GCSE Chemistry My Revision Notes
... (a) Suggest one reason why this part of Newlands’ table is different from the modern one. (1 mark) In 1869 Dimitri Mendeleev arranged the elements by putting them in order of their atomic weights. When he put them into a table he ensured that elements with similar properties were in columns. (b) Wha ...
... (a) Suggest one reason why this part of Newlands’ table is different from the modern one. (1 mark) In 1869 Dimitri Mendeleev arranged the elements by putting them in order of their atomic weights. When he put them into a table he ensured that elements with similar properties were in columns. (b) Wha ...
H - Quantum Condensed Matter Research Group
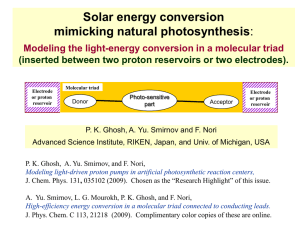
... J. Phys. Chem. C 113, 21218 (2009). Complimentary color copies of these are online. ...
... J. Phys. Chem. C 113, 21218 (2009). Complimentary color copies of these are online. ...
UNITS OF CONCENTRATION
... e.g., mg/L of NO3- (mass of nitrate ions per liter) Or in terms of a particular element in a species that was measured. e.g., mg/L of NO3- - N (mass of nitrogen in the form of nitrate ions per liter) To convert from one to the other of these, use the molar mass ratio of the element to that of the ch ...
... e.g., mg/L of NO3- (mass of nitrate ions per liter) Or in terms of a particular element in a species that was measured. e.g., mg/L of NO3- - N (mass of nitrogen in the form of nitrate ions per liter) To convert from one to the other of these, use the molar mass ratio of the element to that of the ch ...
Regents Chemistry Review - New York Science Teacher
... • Which of the following represents the molecular formula of the organic product in the equation? (1) C4H7COOC2H5 ...
... • Which of the following represents the molecular formula of the organic product in the equation? (1) C4H7COOC2H5 ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.