Gas Laws
... 10. CaBr2 + Na2CO3 CaCO3(aq) + NaBr(aq) Chapter 9: Stoichiometry 1. Ideal combustion, resulting in only carbon dioxide and water, rarely happens. In general, at the very least, some carbon monoxide is also produced. The real combustion of methane is more closely represented, then, by the unbalance ...
... 10. CaBr2 + Na2CO3 CaCO3(aq) + NaBr(aq) Chapter 9: Stoichiometry 1. Ideal combustion, resulting in only carbon dioxide and water, rarely happens. In general, at the very least, some carbon monoxide is also produced. The real combustion of methane is more closely represented, then, by the unbalance ...
asian journal of chemistry asian journal of chemistry
... of low heat transfer and easy automation and incident power ...
... of low heat transfer and easy automation and incident power ...
Document
... – The SI definition of the mole is the amount of a substance that contains as many entities as there are in exactly 0.012 kg (12 g) of carbon‐12. – A mole of atoms or molecules is a perfectly manageable quantity to use in a reaction (see Fig. 3.4). – A 12.01‐gram sample of natural carbon contain ...
... – The SI definition of the mole is the amount of a substance that contains as many entities as there are in exactly 0.012 kg (12 g) of carbon‐12. – A mole of atoms or molecules is a perfectly manageable quantity to use in a reaction (see Fig. 3.4). – A 12.01‐gram sample of natural carbon contain ...
19_Worked_Examples
... Plan We expect ΔS to be positive if there is an increase in temperature, increase in volume, or increase in number of gas particles. The question states that the temperature is constant, and so we need to concern ourselves only with volume and number of particles. Solve (a) Evaporation involves a la ...
... Plan We expect ΔS to be positive if there is an increase in temperature, increase in volume, or increase in number of gas particles. The question states that the temperature is constant, and so we need to concern ourselves only with volume and number of particles. Solve (a) Evaporation involves a la ...
students` errors in solving numerical chemical
... most complex and difficult general chemistry problems. It is not then surprising that many researchers have dealt with them from a number of perspectives. Camacho and Good (1989) studied the problem-solving behaviours of experts and novices engaged in solving chemicalequilibrium problems, and report ...
... most complex and difficult general chemistry problems. It is not then surprising that many researchers have dealt with them from a number of perspectives. Camacho and Good (1989) studied the problem-solving behaviours of experts and novices engaged in solving chemicalequilibrium problems, and report ...
HONORS LAB MANUAL - Tenafly High School
... 1. In addition to the magnesium oxide, a small amount of another compound of magnesium forms during the heating in air. In step 5, water was added to the contents in the crucible to convert this compound into magnesium oxide. The odor indicated that ammonia was also formed in the reaction with water ...
... 1. In addition to the magnesium oxide, a small amount of another compound of magnesium forms during the heating in air. In step 5, water was added to the contents in the crucible to convert this compound into magnesium oxide. The odor indicated that ammonia was also formed in the reaction with water ...
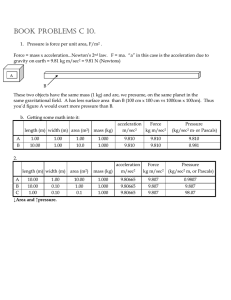
book problems c 10.
... in 1010. The relative atomic mass of silicon is particularly important, since silicon crystals are used in the x-ray methods mentioned above. As a continuation of this approach, one of the 1999 NIST Precision Measurement Grants has been awarded to David Pritchard, physics professor at the Massachuse ...
... in 1010. The relative atomic mass of silicon is particularly important, since silicon crystals are used in the x-ray methods mentioned above. As a continuation of this approach, one of the 1999 NIST Precision Measurement Grants has been awarded to David Pritchard, physics professor at the Massachuse ...
(a) From , 2012 General Chemistry I
... Suppose that 1.00 mol of ideal gas molecules maintained at 292 K and 3.00 atm expands from 8.00 L to 20.00 L and a final pressure of 1.20 atm by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until its ...
... Suppose that 1.00 mol of ideal gas molecules maintained at 292 K and 3.00 atm expands from 8.00 L to 20.00 L and a final pressure of 1.20 atm by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until its ...
Physical Chemistry Problems. ©Mike Lyons 2009
... Answer either : part (a) and part (b) or part (c) and part (d). a. What is the internal energy U and the enthalpy H of a system? Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed b ...
... Answer either : part (a) and part (b) or part (c) and part (d). a. What is the internal energy U and the enthalpy H of a system? Write down an expression for the First Law of Thermodynamics which relates the change in internal energy of a system to the work done on the system and the heat absorbed b ...
OXIDATION NUMBERS
... In this gas electrode, hydrogen at a pressure of 1 bar is bubbled over a strip of platinum coated in platinum black and immersed in an acid solution containing a hydrogen ion concentration of 1.0 mol.dm-3. Temperature = 298K The following equilibrium is established: H+(aq) + e- ...
... In this gas electrode, hydrogen at a pressure of 1 bar is bubbled over a strip of platinum coated in platinum black and immersed in an acid solution containing a hydrogen ion concentration of 1.0 mol.dm-3. Temperature = 298K The following equilibrium is established: H+(aq) + e- ...
Moles 2016
... It started with the atomic masses for the elements. For example, carbon has an average atomic mass of 12.01 amu, meaning that one atom of carbon has a mass of 12.01 amu. The scientists wanted to be able to use the convenient atomic masses for the elements, but on a larger scale. So they decide ...
... It started with the atomic masses for the elements. For example, carbon has an average atomic mass of 12.01 amu, meaning that one atom of carbon has a mass of 12.01 amu. The scientists wanted to be able to use the convenient atomic masses for the elements, but on a larger scale. So they decide ...
Chemistry (306) - National Evaluation Series
... A gas occupies a volume of 1.25 liters at a pressure of 825 mm Hg. What will be the final pressure of this gas if it is compressed into a volume of 725 mL at constant temperature? A. 479 mm Hg B. 748 mm Hg C. 1.30 × 103 mm Hg D. 1.42 × 103 mm Hg ...
... A gas occupies a volume of 1.25 liters at a pressure of 825 mm Hg. What will be the final pressure of this gas if it is compressed into a volume of 725 mL at constant temperature? A. 479 mm Hg B. 748 mm Hg C. 1.30 × 103 mm Hg D. 1.42 × 103 mm Hg ...
Balancing Oxidation-Reduction Equations
... choice of equation type (molecular, ionic, or net ionic) in terms of utility for the given circumstances. LO 3.10: The student is able to evaluate the classification of a process as a physical change, chemical change, or ambiguous change based on both macroscopic observations and the distinction bet ...
... choice of equation type (molecular, ionic, or net ionic) in terms of utility for the given circumstances. LO 3.10: The student is able to evaluate the classification of a process as a physical change, chemical change, or ambiguous change based on both macroscopic observations and the distinction bet ...
A) I is TRUE, II is FALSE B) I is FALSE, II is TRUE C) I and II
... is exothermic, with an absorption of energy is endothermic, with an absorption of energy can be either exothermic or endothermic ...
... is exothermic, with an absorption of energy is endothermic, with an absorption of energy can be either exothermic or endothermic ...
Carboxylic Acid Derivatives and Nitrogen Cpds
... Trichloroethanal (chloral), CCl3CHO, was much used up to the middle of this century as a drug and a stimulant. ...
... Trichloroethanal (chloral), CCl3CHO, was much used up to the middle of this century as a drug and a stimulant. ...
Chapter 3 - Higher Education | Kendall Hunt Publishing
... in the mass of the substances is observed. In other words, mass is neither created nor destroyed in an ordinary chemical reaction. This law has been tested by extensive experimentation in the laboratory, and the work of the brilliant French chemist-physicist Antoine Lavoisier provides evidence for t ...
... in the mass of the substances is observed. In other words, mass is neither created nor destroyed in an ordinary chemical reaction. This law has been tested by extensive experimentation in the laboratory, and the work of the brilliant French chemist-physicist Antoine Lavoisier provides evidence for t ...
Chapter 9 Gases worksheet
... 10. Go through Sample Exercises 9.6 and 9.7 including the Practice Exercises for both these problems. 11. You must be able to carry out calculations in gas chemical reactions and apply the law of combining volumes. 12. Go through Sample Exercise 9.8 including the Practice Exercise. 13. You must unde ...
... 10. Go through Sample Exercises 9.6 and 9.7 including the Practice Exercises for both these problems. 11. You must be able to carry out calculations in gas chemical reactions and apply the law of combining volumes. 12. Go through Sample Exercise 9.8 including the Practice Exercise. 13. You must unde ...
chapter 4 - reactions in solution
... 1. Most salts containing Na+, K+, and NH4+ are soluble. 1. Most compounds of nitrate (NO3-) are soluble. 3. Most salts containing Cl-, Br-, and I- ions are soluble, except the following compounds: AgCl, AgBr, AgI, PbCl2, PbBr2, PbI2), and Hg2Cl2, Hg2Br2, Hg2I2, and HgI2 . 4. Most sulfate salts are s ...
... 1. Most salts containing Na+, K+, and NH4+ are soluble. 1. Most compounds of nitrate (NO3-) are soluble. 3. Most salts containing Cl-, Br-, and I- ions are soluble, except the following compounds: AgCl, AgBr, AgI, PbCl2, PbBr2, PbI2), and Hg2Cl2, Hg2Br2, Hg2I2, and HgI2 . 4. Most sulfate salts are s ...
California Standards Practice - Student Edition
... conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe chemical reactions by writing balanced equations. b. Students know the quantity one mole is set by defining one mole of carbon 12 atoms ...
... conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe chemical reactions by writing balanced equations. b. Students know the quantity one mole is set by defining one mole of carbon 12 atoms ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.