Chapter 3
... – how much reactant is consumed and how much product is formed – coefficients must be consistent with the Law of Conservation of Mass; atoms are neither created nor destroyed in a chemical reaction. – i.e. chemical equation must be balanced ...
... – how much reactant is consumed and how much product is formed – coefficients must be consistent with the Law of Conservation of Mass; atoms are neither created nor destroyed in a chemical reaction. – i.e. chemical equation must be balanced ...
PRACTICE * Naming and Writing Ionic Compounds
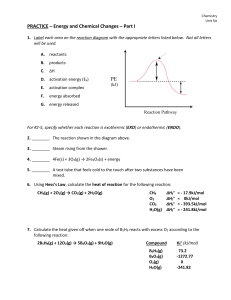
... 2. ________ The reaction shown in the diagram above. 3. ________ Steam rising from the shower. 4. ________ 4Fe(s) + 3O2(g) → 2Fe2O3(s) + energy 5. ________ A test tube that feels cold to the touch after two substances have been mixed. 6. Using Hess’s Law, calculate the heat of reaction for the follo ...
... 2. ________ The reaction shown in the diagram above. 3. ________ Steam rising from the shower. 4. ________ 4Fe(s) + 3O2(g) → 2Fe2O3(s) + energy 5. ________ A test tube that feels cold to the touch after two substances have been mixed. 6. Using Hess’s Law, calculate the heat of reaction for the follo ...
Les Équations Chimiques
... Writing Chemical Equations The simplest form of a chemical equation is called the nominative equation (in this type of equation we use words, not symbols) ...
... Writing Chemical Equations The simplest form of a chemical equation is called the nominative equation (in this type of equation we use words, not symbols) ...
Reaction Stoichiometry
... be the same on both sides of a balanced equation. Subscripts must not be changed to balance an equation. A balanced equation tells us the ratio of the number of molecules which react and are produced in a chemical reaction. Coefficients can be fractions, although they are usually given as lowest int ...
... be the same on both sides of a balanced equation. Subscripts must not be changed to balance an equation. A balanced equation tells us the ratio of the number of molecules which react and are produced in a chemical reaction. Coefficients can be fractions, although they are usually given as lowest int ...
Ch. 3 - Chemical Reactions
... Describing Equations Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) • How many? • Of what? • In what state? ...
... Describing Equations Zn(s) + 2HCl(aq) ZnCl2(aq) + H2(g) • How many? • Of what? • In what state? ...
Chemical Reactions
... Synthesis Reaction • Synthesis – 2 substances (reactants) combine to form a new substance (product). – Substances are either atoms (elements) or compounds in this case. A + ...
... Synthesis Reaction • Synthesis – 2 substances (reactants) combine to form a new substance (product). – Substances are either atoms (elements) or compounds in this case. A + ...
File
... “Exothermic” means that heat is released during the reaction. This often results in the reaction container feeling warm to the touch (heat is given off). Reactants Products + HEAT (heat on product side because released) “Endothermic” means that heat is absorbed during the reaction. This often resu ...
... “Exothermic” means that heat is released during the reaction. This often results in the reaction container feeling warm to the touch (heat is given off). Reactants Products + HEAT (heat on product side because released) “Endothermic” means that heat is absorbed during the reaction. This often resu ...
Chapter 6-student notes
... a representation of a chemical reaction in which the formulas of the reactants and products are used instead of the names of the compounds. Reactants are still separated from each other by a + and reactants and products are separated by an arrow Example: CH4 +O2 -> CO2 +H2O Try these: Change the ...
... a representation of a chemical reaction in which the formulas of the reactants and products are used instead of the names of the compounds. Reactants are still separated from each other by a + and reactants and products are separated by an arrow Example: CH4 +O2 -> CO2 +H2O Try these: Change the ...
Chemical Reactions
... • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as the law of conservation of mass ...
... • The principle that during chemical reactions, the mass of the products is always equal to the mass of the reactants, is known as the law of conservation of mass ...
Name
... 21. In what units is molar mass typically expressed? a. Kg b. L c. Amu d. g/mol 22. The sum of the percentages in the percentage composition of a substance equals a. 100. b. the molar mass. c. the molar volume. d. Avogadro’s number. 23. The simplest whole-number ratio of the atoms of the elements in ...
... 21. In what units is molar mass typically expressed? a. Kg b. L c. Amu d. g/mol 22. The sum of the percentages in the percentage composition of a substance equals a. 100. b. the molar mass. c. the molar volume. d. Avogadro’s number. 23. The simplest whole-number ratio of the atoms of the elements in ...
Ch.5
... If 18.0 g hydrogen peroxide react with the amount of dinitrogen tetrahydride determined in Q.#3 and produces 15.6 g water, what is the percent yield? ...
... If 18.0 g hydrogen peroxide react with the amount of dinitrogen tetrahydride determined in Q.#3 and produces 15.6 g water, what is the percent yield? ...
The only sure evidence that a chemical reaction has occured is
... 3. A shorter, easier way to show chemical reactions, using symbols instead of words, is called a _____. 4. The substances on the left of the arrow in a chemical equation are the substances you start with called ______. 5. Give an example of a change that is NOT a chemical reaction? 6. How many atoms ...
... 3. A shorter, easier way to show chemical reactions, using symbols instead of words, is called a _____. 4. The substances on the left of the arrow in a chemical equation are the substances you start with called ______. 5. Give an example of a change that is NOT a chemical reaction? 6. How many atoms ...
Final Exam Study Guide Page 1 Quiz
... a. Is completely used up in the reaction b. Will have some amount unchanged, or leftover, after the reaction c. Cannot be calculated without performing the reaction d. Has no effect in the amount of product formed ...
... a. Is completely used up in the reaction b. Will have some amount unchanged, or leftover, after the reaction c. Cannot be calculated without performing the reaction d. Has no effect in the amount of product formed ...
Key To T2 Review For Final Study Guide File - District 196 e
... 12. Using dimensional analysis, convert the following: a. 2.00 moles of oxygen gas to grams 64.0 g O2 b. 6.75 x 1022 molecules of oxygen gas to grams 3.59 g O2 c. 4.66 g iodine crystals to molecules 1.11 x 1022 molecules I2 d. 7.6 x 1023 formula units of salt to moles 1.3 mol NaCl 13. What is the di ...
... 12. Using dimensional analysis, convert the following: a. 2.00 moles of oxygen gas to grams 64.0 g O2 b. 6.75 x 1022 molecules of oxygen gas to grams 3.59 g O2 c. 4.66 g iodine crystals to molecules 1.11 x 1022 molecules I2 d. 7.6 x 1023 formula units of salt to moles 1.3 mol NaCl 13. What is the di ...
1 Types of Chemical Reactions
... A colour change occurs. A new gas with different properties is formed. Sudden dramatic changes in ...
... A colour change occurs. A new gas with different properties is formed. Sudden dramatic changes in ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.