Exam 2
... • Write your student number in the space provided above on this page. • Check that your name and student number as printed on your answer sheet for multiple-choice questions are correct, and sign your name in the space provided to verify this. • All written responses must be in English. At the end o ...
... • Write your student number in the space provided above on this page. • Check that your name and student number as printed on your answer sheet for multiple-choice questions are correct, and sign your name in the space provided to verify this. • All written responses must be in English. At the end o ...
File - Mc Guckin Science
... b) C2H6 + O2 CO2 + H2O _____________________ c) MgO + H3PO4 Mg3(PO4)2 + H2O _____________________ d) PbO2 PbO + O2 _____________________ e) SiO2 + HF SiF4 + H2O _____________________ f) C10H22 + O2 CO2 + H2O _____________________ g) Mg + H2SO4 H2 + MgSO4 _____________________ h) Sb2S3 + HC ...
... b) C2H6 + O2 CO2 + H2O _____________________ c) MgO + H3PO4 Mg3(PO4)2 + H2O _____________________ d) PbO2 PbO + O2 _____________________ e) SiO2 + HF SiF4 + H2O _____________________ f) C10H22 + O2 CO2 + H2O _____________________ g) Mg + H2SO4 H2 + MgSO4 _____________________ h) Sb2S3 + HC ...
prs-A3
... The following diagram represents the collection of carbon dioxide and water formed by the decomposition of a hydrocarbon. What was the empirical formula of the original hydrocarbon? • C4H16 • C 2H 8 • CH4 • While the diagram indicates 4 carbons, and you might think there could have been 1 C4H16, 2 ...
... The following diagram represents the collection of carbon dioxide and water formed by the decomposition of a hydrocarbon. What was the empirical formula of the original hydrocarbon? • C4H16 • C 2H 8 • CH4 • While the diagram indicates 4 carbons, and you might think there could have been 1 C4H16, 2 ...
The Mole - cloudfront.net
... A formula which gives the lowest whole-number ratio of the atoms of the elements in a compound. • The percent composition is required to calculate the basic ratio of the elements contained in a compound. • The empirical formula, like any chemical formula, may be interpreted in terms of number of ato ...
... A formula which gives the lowest whole-number ratio of the atoms of the elements in a compound. • The percent composition is required to calculate the basic ratio of the elements contained in a compound. • The empirical formula, like any chemical formula, may be interpreted in terms of number of ato ...
UNIT 5 - H-W Science Website
... 4(C-H) + 2(O=O) = 4(414 kJ) + 2(499 kJ) = energy to break bonds (+) and 2(C=O) + 4(H-O) = 2(799 kJ) + 4(460 kJ) = energy to make bonds (-) The sum of these two values is -784 kJ--in other words, 784 kJ is released when one mole of methane is burned. The experimental heat of combustion is -803 kJ. So ...
... 4(C-H) + 2(O=O) = 4(414 kJ) + 2(499 kJ) = energy to break bonds (+) and 2(C=O) + 4(H-O) = 2(799 kJ) + 4(460 kJ) = energy to make bonds (-) The sum of these two values is -784 kJ--in other words, 784 kJ is released when one mole of methane is burned. The experimental heat of combustion is -803 kJ. So ...
Equilibrium and Pressure
... eventually result in chemical equilibrium. Chemical equilibrium is reached when the rates of the forward and reverse reactions are the same. The constant Kc describes the ratio of products to reactants at equilibrium. A similar equilibrium can be calculated based on partial pressure. Question: How c ...
... eventually result in chemical equilibrium. Chemical equilibrium is reached when the rates of the forward and reverse reactions are the same. The constant Kc describes the ratio of products to reactants at equilibrium. A similar equilibrium can be calculated based on partial pressure. Question: How c ...
Chapter 4: Chemical Reaction Dynamics
... be measured in crossed molecular beam experiments. The angular distribution of the scattering products is measured with a moveable detector in the laboratory frame. The distribution of scattering angles θ and product velocities uAB in the centre-of-mass (COM) frame can be inferred from a Newton diag ...
... be measured in crossed molecular beam experiments. The angular distribution of the scattering products is measured with a moveable detector in the laboratory frame. The distribution of scattering angles θ and product velocities uAB in the centre-of-mass (COM) frame can be inferred from a Newton diag ...
Matter and Change
... • What is the difference between an element and a pure substance? • Provide an example of an element and a compound. • Where would you find a list of elements? ...
... • What is the difference between an element and a pure substance? • Provide an example of an element and a compound. • Where would you find a list of elements? ...
BIOL 157 * BIOLOGICAL CHEMISTRY Lecture 6
... determined by titration with sodium thiosulphate, Na2S2O3, using starch as the indicator. • Assuming that the concentration of iodine formed in 30 ...
... determined by titration with sodium thiosulphate, Na2S2O3, using starch as the indicator. • Assuming that the concentration of iodine formed in 30 ...
Enzyme Activity
... Many are a lot lower, cold water fish will die at 30°C because their enzymes denature A few bacteria have enzymes very high temperatures up to 100°C Most enzymes however are fully denatured at 70°C ...
... Many are a lot lower, cold water fish will die at 30°C because their enzymes denature A few bacteria have enzymes very high temperatures up to 100°C Most enzymes however are fully denatured at 70°C ...
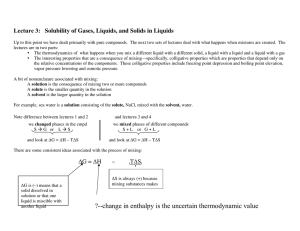
LIQUIDS
... When two non-metal atoms combine they both need to gain electrons, and they can do this by sharing two electrons (normally one from each atom) in a covalent bond. A COVALENT BOND is defined as; the electrostatic attraction between the positively charged protons in the nucleus and the negative shared ...
... When two non-metal atoms combine they both need to gain electrons, and they can do this by sharing two electrons (normally one from each atom) in a covalent bond. A COVALENT BOND is defined as; the electrostatic attraction between the positively charged protons in the nucleus and the negative shared ...
1. Natures Chemistry Unit Questions
... forms a bond with the carbonyl carbon atom of the second molecule. (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde that would not take part in an aldol condensation. (1) (c) Apart from the structure of ...
... forms a bond with the carbonyl carbon atom of the second molecule. (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde that would not take part in an aldol condensation. (1) (c) Apart from the structure of ...
Redox Reactions and Electrochemistry
... balanced. The next step is to combine the two halfreactions to form an overall equation. 6) Multiply through each half-reactions by appropriate coefficients to match electrons in each half-reaction. (i.e. number of electrons lost by the oxidized species must equal the number gained by the reduced on ...
... balanced. The next step is to combine the two halfreactions to form an overall equation. 6) Multiply through each half-reactions by appropriate coefficients to match electrons in each half-reaction. (i.e. number of electrons lost by the oxidized species must equal the number gained by the reduced on ...
Chemistry 12 - hrsbstaff.ednet.ns.ca
... A. The decomposition of KBr(s) is an endothermic process. B. The dissolving of KBr(s) is an exothermic process. C. The equation represents a phase change. D. KBr(s) is less stable than its constituent elements. 7. What is the enthalpy change for the following reaction? 2SO2(g) + O2(g) 2SO3(g) A. - ...
... A. The decomposition of KBr(s) is an endothermic process. B. The dissolving of KBr(s) is an exothermic process. C. The equation represents a phase change. D. KBr(s) is less stable than its constituent elements. 7. What is the enthalpy change for the following reaction? 2SO2(g) + O2(g) 2SO3(g) A. - ...
Energetics - WordPress.com
... This is interesting not only because it is a reaction between two solids, but also because the temperature decrease is such that the flask will often stick to the bench after the reaction. When there is a change of state from solid to a liquid, or a liquid to a gas at a constant temperature, the ...
... This is interesting not only because it is a reaction between two solids, but also because the temperature decrease is such that the flask will often stick to the bench after the reaction. When there is a change of state from solid to a liquid, or a liquid to a gas at a constant temperature, the ...
Reactions of Metals and Their Compounds
... carbon dioxide(CO2) • Barium chloride (BaCl2) + Sulphuric acid (H2SO4) → Barium sulphate (BaSO4) + Hydrochloric acid (HCl) ...
... carbon dioxide(CO2) • Barium chloride (BaCl2) + Sulphuric acid (H2SO4) → Barium sulphate (BaSO4) + Hydrochloric acid (HCl) ...
Mass # = Atomic # + # Neutrons
... to the number of electrons, atomic number also indicates the number of electrons in a single atom of an element. The Periodic Table is arranged according to atomic number. For example, hydrogen is element 1 and has 1 proton in its nucleus. Similarly, helium is element 2 and has 2 protons in its nucl ...
... to the number of electrons, atomic number also indicates the number of electrons in a single atom of an element. The Periodic Table is arranged according to atomic number. For example, hydrogen is element 1 and has 1 proton in its nucleus. Similarly, helium is element 2 and has 2 protons in its nucl ...
2006 Practice Final Exam - Department of Chemistry | Oregon State
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your test form number (listed above), l ...
Balancing Redox Equations
... Oxidizing agent when it combines with metals. Reducing agent when it combines with nonmetals. ...
... Oxidizing agent when it combines with metals. Reducing agent when it combines with nonmetals. ...
Course Pack3 Phase Diagrams
... The rule you always hear about miscibility is “like dissolves like” which is an easy way of saying that if the intermolecular forces (IMF) are alike, then compounds are miscible and if the IMFs are not alike, they are not miscible (immiscible). The explanation is that if you are replacing one form o ...
... The rule you always hear about miscibility is “like dissolves like” which is an easy way of saying that if the intermolecular forces (IMF) are alike, then compounds are miscible and if the IMFs are not alike, they are not miscible (immiscible). The explanation is that if you are replacing one form o ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.