WRITING AP EQUATIONS AP equation sets are found in the free
... anything about acidic or basic solution, it is redox. If you are totally stuck, look up the compounds in the index of your book or other reference books and try to find information that will help you with the equation. All reactions do not fit neatly into the five types of reactions that you learned ...
... anything about acidic or basic solution, it is redox. If you are totally stuck, look up the compounds in the index of your book or other reference books and try to find information that will help you with the equation. All reactions do not fit neatly into the five types of reactions that you learned ...
Redox Reactions and Electrochemistry
... balanced. The next step is to combine the two halfreactions to form an overall equation. 6) Multiply through each half-reactions by appropriate coefficients to match electrons in each half-reaction. (i.e. number of electrons lost by the oxidized species must equal the number gained by the reduced on ...
... balanced. The next step is to combine the two halfreactions to form an overall equation. 6) Multiply through each half-reactions by appropriate coefficients to match electrons in each half-reaction. (i.e. number of electrons lost by the oxidized species must equal the number gained by the reduced on ...
CH 4 Notes
... In general, an acid and base react to form a salt. A salt is any ionic compound whose cation comes from a base, and anion from an acid. The other product, H2O, is a common weak electrolyte. Typical examples of neutralization reactions: Reactions between an acid and a metal hydroxide. Mg(OH)2 (mi ...
... In general, an acid and base react to form a salt. A salt is any ionic compound whose cation comes from a base, and anion from an acid. The other product, H2O, is a common weak electrolyte. Typical examples of neutralization reactions: Reactions between an acid and a metal hydroxide. Mg(OH)2 (mi ...
Reactions of common metals and properties of
... The mechanism of the reaction involves taking the two electrons away from the barium and this requires about 500 kJ mol-1 for the first electron, and 1000 kJ mol-1 for the second. The rate of reaction of the group 2 elements with water is much slower than that of the alkali metals, even in the case ...
... The mechanism of the reaction involves taking the two electrons away from the barium and this requires about 500 kJ mol-1 for the first electron, and 1000 kJ mol-1 for the second. The rate of reaction of the group 2 elements with water is much slower than that of the alkali metals, even in the case ...
Addition of ketene to ethylene oxide
... intermediate in the preparation of surface active agents • . This involves a reaction with long chain carboxylic acids to produce a molecule containing both hydrophobic and hydrophilic characteristics. Ethylene oxide undergoes attack by substances containing active hydrogen and also by some substanc ...
... intermediate in the preparation of surface active agents • . This involves a reaction with long chain carboxylic acids to produce a molecule containing both hydrophobic and hydrophilic characteristics. Ethylene oxide undergoes attack by substances containing active hydrogen and also by some substanc ...
Chemistry Lesson 10 Describing Matter
... 5. Malleability – ability to be hammered or beaten into thin sheets – example: silver is quite malleable, so it is used to make jewelry. ...
... 5. Malleability – ability to be hammered or beaten into thin sheets – example: silver is quite malleable, so it is used to make jewelry. ...
Catalysis
... The active site has a rigid structure, similar to a lock. A substrate molecule has a complementary structure that causes it to fit and function like a key Geometry of an active site only fit one type of substrate in most cases. That’s why enzymes are highly specific in action ...
... The active site has a rigid structure, similar to a lock. A substrate molecule has a complementary structure that causes it to fit and function like a key Geometry of an active site only fit one type of substrate in most cases. That’s why enzymes are highly specific in action ...
Chemical Formula Detective
... Different elements can form chemical bonds to create compounds. For example, sodium and chlorine combine to form sodium chloride, NaCl. In the chemical formula NaCl, there is a 1:1 ratio of sodium ions:chloride ions. However, not all compounds form in a 1:1 ratio of their constituent elements. If th ...
... Different elements can form chemical bonds to create compounds. For example, sodium and chlorine combine to form sodium chloride, NaCl. In the chemical formula NaCl, there is a 1:1 ratio of sodium ions:chloride ions. However, not all compounds form in a 1:1 ratio of their constituent elements. If th ...
22017Stoichiometry
... WE will start stoichiometry today with mole ratio and mole to mole calculations You will get your tests back on Thursday and we will discuss the results 4th period: you can use one of my calculators (AS LONG ...
... WE will start stoichiometry today with mole ratio and mole to mole calculations You will get your tests back on Thursday and we will discuss the results 4th period: you can use one of my calculators (AS LONG ...
Thermodynamics
... when one mole of a compound is formed at 1.0 atm pressure and 25 °C from its elements under the same conditions. ...
... when one mole of a compound is formed at 1.0 atm pressure and 25 °C from its elements under the same conditions. ...
35 - TAMU Chemistry
... (this is a filter used to neutralize any acids that may form during storage) TNT – trinitrotoluene (solid) C7H5N3O6 (s) → huge 3N2 + 7CO2 + 5H2O + 7C(s) entropy (15 moles of gas) increase ...
... (this is a filter used to neutralize any acids that may form during storage) TNT – trinitrotoluene (solid) C7H5N3O6 (s) → huge 3N2 + 7CO2 + 5H2O + 7C(s) entropy (15 moles of gas) increase ...
Amount of Substance
... proton and 1 electron. Since the mass of an electron is negligible compared to that of a proton or a neutron, the hydrogen atom has only 1/12 the mass of a carbon atom; therefore the relative atomic mass of hydrogen is 1. Similarly, the relative mass of an oxygen atom, which contains 8 protons, 8 ne ...
... proton and 1 electron. Since the mass of an electron is negligible compared to that of a proton or a neutron, the hydrogen atom has only 1/12 the mass of a carbon atom; therefore the relative atomic mass of hydrogen is 1. Similarly, the relative mass of an oxygen atom, which contains 8 protons, 8 ne ...
Chem 11 Notes Booklet (pdf version)
... If the zeros in the number were actually measured, they must be shown to be significant. This is done by marking them with a bar or a decimal point. ...
... If the zeros in the number were actually measured, they must be shown to be significant. This is done by marking them with a bar or a decimal point. ...
File
... b) C2H6 + O2 CO2 + H2O _____________________ c) MgO + H3PO4 Mg3(PO4)2 + H2O _____________________ d) PbO2 PbO + O2 _____________________ e) SiO2 + HF SiF4 + H2O _____________________ f) C10H22 + O2 CO2 + H2O _____________________ g) Mg + H2SO4 H2 + MgSO4 _____________________ h) Sb2S3 + HC ...
... b) C2H6 + O2 CO2 + H2O _____________________ c) MgO + H3PO4 Mg3(PO4)2 + H2O _____________________ d) PbO2 PbO + O2 _____________________ e) SiO2 + HF SiF4 + H2O _____________________ f) C10H22 + O2 CO2 + H2O _____________________ g) Mg + H2SO4 H2 + MgSO4 _____________________ h) Sb2S3 + HC ...
Answer
... Chatelier’s principle, the reaction will shift to increase the pressure. It does this by favouring the side with a greater number of gaseous molecules: The reaction will shift to the left (3 moles of gas on the left, 2 moles of gas on the right). Calculate the value of the equilibrium constant, K, a ...
... Chatelier’s principle, the reaction will shift to increase the pressure. It does this by favouring the side with a greater number of gaseous molecules: The reaction will shift to the left (3 moles of gas on the left, 2 moles of gas on the right). Calculate the value of the equilibrium constant, K, a ...
Chapter 4. Aqueous Reactions and Solution Stoichiometry
... • Take a known volume of the HCl solution (i.e., 20.00 mL) and measure the number of mL of 0.100 M NaOH solution required to react completely with the HCl solution. • The point at which stoichiometrically equivalent quantities of NaOH and HCl are brought together is known as the equivalence point of ...
... • Take a known volume of the HCl solution (i.e., 20.00 mL) and measure the number of mL of 0.100 M NaOH solution required to react completely with the HCl solution. • The point at which stoichiometrically equivalent quantities of NaOH and HCl are brought together is known as the equivalence point of ...
110 EXAM Review MATERIALTro
... b. Heterogeneous mixture has 2 or more physically distinct phases. Examples: ...
... b. Heterogeneous mixture has 2 or more physically distinct phases. Examples: ...
Chemical Reactions Definitions Air Fuel Ratio
... Solving these equations, the balanced chemical reaction is: CH 4 2O2 3.76 N 2 CO2 2 H 2 O 7.52 N 2 ...
... Solving these equations, the balanced chemical reaction is: CH 4 2O2 3.76 N 2 CO2 2 H 2 O 7.52 N 2 ...
Elements, Compounds, and Chemical Equations
... Count the atoms in the reactants and the products. • Count the total number of each type of atom on the reactant (ingredient) side. • Count the total number of each type of atom in the product (what you make) side • If the number of each type of atom matches, the equation is balanced. If the numbers ...
... Count the atoms in the reactants and the products. • Count the total number of each type of atom on the reactant (ingredient) side. • Count the total number of each type of atom in the product (what you make) side • If the number of each type of atom matches, the equation is balanced. If the numbers ...
ch16powerpoint
... Because its 600 bond angles allow poor orbital overlap, its bonds are weak. As a result, it is thermally unstable and rearranges to propene at 10000C via a first-order reaction: • The rate constant is 9.2s-1; (a) What is the half-life of the reaction? (b) How long does it take for the concentration ...
... Because its 600 bond angles allow poor orbital overlap, its bonds are weak. As a result, it is thermally unstable and rearranges to propene at 10000C via a first-order reaction: • The rate constant is 9.2s-1; (a) What is the half-life of the reaction? (b) How long does it take for the concentration ...
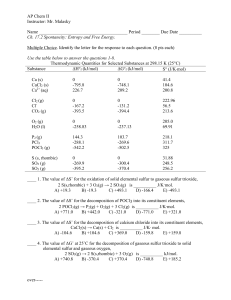
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.