5 Bose-Einstein condensate (BEC)
... interactions between the species. We propose two different schemes for creating these correlations: a dynamical scheme and a static scheme analogous to two-mode squeezing in quantum optics. We quantify the correlations by using known measures of entanglement and study the effect of finite temperature o ...
... interactions between the species. We propose two different schemes for creating these correlations: a dynamical scheme and a static scheme analogous to two-mode squeezing in quantum optics. We quantify the correlations by using known measures of entanglement and study the effect of finite temperature o ...
The Quantum Mechanical Picture of the Atom
... n - the principal quantum number n = 1, 2, 3, 4, ...... ...
... n - the principal quantum number n = 1, 2, 3, 4, ...... ...
Research Status, Winter 2009 - Cove
... – Need to go from 0 to n2 , this is a huge number of calculations for a 128 bit number. This means 2(2*128) or ~1.16 x 1077 – The results have to be stored somewhere (taking up memory) and then we still have find the period! – Or we can just use 384 qubits and run through a set of quantum operations ...
... – Need to go from 0 to n2 , this is a huge number of calculations for a 128 bit number. This means 2(2*128) or ~1.16 x 1077 – The results have to be stored somewhere (taking up memory) and then we still have find the period! – Or we can just use 384 qubits and run through a set of quantum operations ...
Models of the Atom
... It correctly predicts the spectral series for hydrogen, but fails predicting the same for atoms with 2 or more electrons. A more general approach was developed in 1925/6 by Erwin Schrodinger, Werner Heisenberg, and others, and is called quantum mechanics. ...
... It correctly predicts the spectral series for hydrogen, but fails predicting the same for atoms with 2 or more electrons. A more general approach was developed in 1925/6 by Erwin Schrodinger, Werner Heisenberg, and others, and is called quantum mechanics. ...
Introduction to Nanoelectronics Marc Baldo MIT OpenCourseWare Publication May 2011
... presentation by Supriyo Datta from Purdue. He was describing electronic devices from the „bottom up‟ – starting with quantum mechanical descriptions of atoms and molecules, and ending up with device-scale current-voltage characteristics. Although I did not understand the details at the time, it was ...
... presentation by Supriyo Datta from Purdue. He was describing electronic devices from the „bottom up‟ – starting with quantum mechanical descriptions of atoms and molecules, and ending up with device-scale current-voltage characteristics. Although I did not understand the details at the time, it was ...
Section 4.2 The Quantum Model of the Atom
... To define the region in which electrons can be found, scientists have assigned four quantum numbers that specify the properties of the electrons. ...
... To define the region in which electrons can be found, scientists have assigned four quantum numbers that specify the properties of the electrons. ...
Document
... The answer at which we arrive is the wave-function should not be regarded as a complete description of the physical state of the system. We consider a composite system, consisting of the partial systems A and B which interact for a short time only. We assume that we know the wave-function of the com ...
... The answer at which we arrive is the wave-function should not be regarded as a complete description of the physical state of the system. We consider a composite system, consisting of the partial systems A and B which interact for a short time only. We assume that we know the wave-function of the com ...
Slides from Lecture 9-11
... pattern: vector approach avoids calculating wave functions when not needed. Wave function picture incomplete: If you know ψ(r) you know everything about: position, momentum, KE, orbital angular momentum …but nothing about spin (+ other more obscure quantities) ...
... pattern: vector approach avoids calculating wave functions when not needed. Wave function picture incomplete: If you know ψ(r) you know everything about: position, momentum, KE, orbital angular momentum …but nothing about spin (+ other more obscure quantities) ...
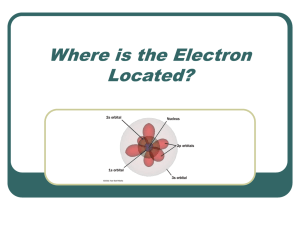
Where is the Electron Located?
... electrons per orbital and they must spin in opposite directions Hund’s Rule: Each orbital of equal energy must have one electron before a second electron is added ...
... electrons per orbital and they must spin in opposite directions Hund’s Rule: Each orbital of equal energy must have one electron before a second electron is added ...