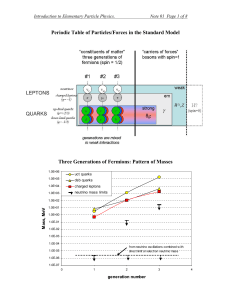
Periodic Table of Particles/Forces in the Standard Model
... quantum numbers like charge (electric, color, etc.), magnetic moment, etc. For photon , Z, and H, an anti-particle is the same as a particle. Same can be true for neutrinos, but we do not yet know this… In general, fermions—particles with half-integral spin: ½ , 3/2, …. Bosons—particles with integra ...
... quantum numbers like charge (electric, color, etc.), magnetic moment, etc. For photon , Z, and H, an anti-particle is the same as a particle. Same can be true for neutrinos, but we do not yet know this… In general, fermions—particles with half-integral spin: ½ , 3/2, …. Bosons—particles with integra ...
Subject Area Assessment Guides
... metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The transition metals (Groups 3 through 12) are represented by some of the ...
... metals (Group 1), such as sodium and potassium, which are soft and white and extremely reactive chemically. Alkaline earth metals (Group 2), such as magnesium and calcium, are found in the second column of the periodic table. The transition metals (Groups 3 through 12) are represented by some of the ...
Chem BIG REVIEW - Jones-wiki
... are ductile. Most have extremely high melting points. Reactivity increases as you go down within a group for metals. With metals the greater the tendency to lose electrons, the more reactive the metal is. Reactive metals have low ionization energies and low electronegativities. Most nonmetals don’t ...
... are ductile. Most have extremely high melting points. Reactivity increases as you go down within a group for metals. With metals the greater the tendency to lose electrons, the more reactive the metal is. Reactive metals have low ionization energies and low electronegativities. Most nonmetals don’t ...
Chemistry Definitions
... charge and remove an electron from a gaseous atom. Removing 1 electron results in a +1 charge. Electron affinity: The energy that is required (or given out) to overcome the attraction of the nuclear charge and add an electron to a gaseous atom. Adding 1 electron results in a –1 charge. Orbital: The ...
... charge and remove an electron from a gaseous atom. Removing 1 electron results in a +1 charge. Electron affinity: The energy that is required (or given out) to overcome the attraction of the nuclear charge and add an electron to a gaseous atom. Adding 1 electron results in a –1 charge. Orbital: The ...
Accelerate This! - University of Houston
... Natural Radioactivity Ernest Rutherford used naturally occurring alpha particles with energies of approximately 5 MeV to discover the nucleus. ...
... Natural Radioactivity Ernest Rutherford used naturally occurring alpha particles with energies of approximately 5 MeV to discover the nucleus. ...
LECTURE 14 HADRONS PHY492 Nuclear and Elementary Particle Physics
... PHY492 Nuclear and Elementary Particle Physics ...
... PHY492 Nuclear and Elementary Particle Physics ...
Introduction to Nuclear Radiation
... Counting statistics Read Chapter 11 of "An Introduction to Error Analysis" by John R. Taylor for additional information on counting statistics and the Poisson distribution. The normal or Gaussian distribution is discussed in chapter 5. Since each nucleus emits its radiation independently, the radiat ...
... Counting statistics Read Chapter 11 of "An Introduction to Error Analysis" by John R. Taylor for additional information on counting statistics and the Poisson distribution. The normal or Gaussian distribution is discussed in chapter 5. Since each nucleus emits its radiation independently, the radiat ...
Nick Childs - Physics
... forever. Prior to his analysis of alpha particles incident on gold foil, the atom was thought of as a “plum pudding” in which electrons, the plums, resided in a pudding of positive charge. The experiments conducted starting in 1909 by Hans Geiger and Ernest Marsden, under the supervision of Ernest R ...
... forever. Prior to his analysis of alpha particles incident on gold foil, the atom was thought of as a “plum pudding” in which electrons, the plums, resided in a pudding of positive charge. The experiments conducted starting in 1909 by Hans Geiger and Ernest Marsden, under the supervision of Ernest R ...
Electricity and Magnetism
... electrons. An object is electrically charged if the numbers of protons and electrons are not equal. ...
... electrons. An object is electrically charged if the numbers of protons and electrons are not equal. ...
Chapter 2 - Molecules of Life (Biochemistry) Periodic Table of
... • Relatively weak bonds, but lots of them together can be strong. ! • Result from unequal sharing of electrons in polar covalent molecules.! • Partial positive and negative charges on different molecules attract each other.! Water is a polar covalent molecule. ! • Electrons are shared unequally ...
... • Relatively weak bonds, but lots of them together can be strong. ! • Result from unequal sharing of electrons in polar covalent molecules.! • Partial positive and negative charges on different molecules attract each other.! Water is a polar covalent molecule. ! • Electrons are shared unequally ...
Matter and Energy Notes
... variable volume Easily compressed Vapor = gaseous state of a substance that is a liquid or solid at room temperature ...
... variable volume Easily compressed Vapor = gaseous state of a substance that is a liquid or solid at room temperature ...
Atomic nucleus
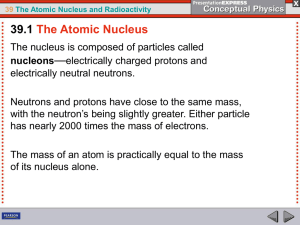
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.