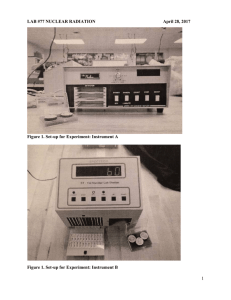
Lab 77 Nuclear Radiation Detection
... are called radioisotopes. All elements with atomic numbers greater than 82 (and some with smaller atomic numbers) possess naturally radioactive isotopes. In addition, artificial radioactive isotopes can be created by bombarding certain stable isotopes with particles. To date, over 2,000 radioisotope ...
... are called radioisotopes. All elements with atomic numbers greater than 82 (and some with smaller atomic numbers) possess naturally radioactive isotopes. In addition, artificial radioactive isotopes can be created by bombarding certain stable isotopes with particles. To date, over 2,000 radioisotope ...
Chemistry - School District of Springfield Township
... o Experiments have revealed that the mass of an atom is concentrated in a tiny positively charged nucleus (consisting of protons and neutrons) with a negative cloud of electrons moving around the nucleus. o The number of protons in the nucleus (and electrons in the cloud) is called the atomic number ...
... o Experiments have revealed that the mass of an atom is concentrated in a tiny positively charged nucleus (consisting of protons and neutrons) with a negative cloud of electrons moving around the nucleus. o The number of protons in the nucleus (and electrons in the cloud) is called the atomic number ...
Summaries of Review Topics for AP Chemistry
... (2) If an ion consists of only one atom, it’s called a monatomic ion. The charge (or oxidation number/state) of a monatomic ion is equal to the number of electrons that were transferred from an atom. Ex: Ca2+ (the Calcium atom lost 2 valence electrons) and S2- (S gained 2 electrons.) (3) An ion that ...
... (2) If an ion consists of only one atom, it’s called a monatomic ion. The charge (or oxidation number/state) of a monatomic ion is equal to the number of electrons that were transferred from an atom. Ex: Ca2+ (the Calcium atom lost 2 valence electrons) and S2- (S gained 2 electrons.) (3) An ion that ...
MISE - Physical Basis of Chemistry
... Up to now, we’ve been talking about relative atomic weights and we have been working in ratio - using the “triangle”. Since individual weights appear in the periodic table, there has to be a mass standard, i.e., a reference mass - so that the ratio of atomic weights can become individual values. Sin ...
... Up to now, we’ve been talking about relative atomic weights and we have been working in ratio - using the “triangle”. Since individual weights appear in the periodic table, there has to be a mass standard, i.e., a reference mass - so that the ratio of atomic weights can become individual values. Sin ...
Concepts in Theoretical Physics
... Why do the quarks stick together in this way? It s because the quarks are the only particles to feel the strong nuclear force. To understand this better, we next need to look at the forces. ...
... Why do the quarks stick together in this way? It s because the quarks are the only particles to feel the strong nuclear force. To understand this better, we next need to look at the forces. ...
Table of Contents - Free Coursework for GCSE, IGCSE, A Level, IB
... First Ionization Energy the energy required to remove one electron from each atom of a mole of atoms in the gas state, to form one mole of cations in the gas phase, under s.t.p X ( g ) X (g ) e ...
... First Ionization Energy the energy required to remove one electron from each atom of a mole of atoms in the gas state, to form one mole of cations in the gas phase, under s.t.p X ( g ) X (g ) e ...
Atomic Theory
... First Ionization Energy the energy required to remove one electron from each atom of a mole of atoms in the gas state, to form one mole of cations in the gas phase, under s.t.p X ( g ) X (g ) e ...
... First Ionization Energy the energy required to remove one electron from each atom of a mole of atoms in the gas state, to form one mole of cations in the gas phase, under s.t.p X ( g ) X (g ) e ...
Atomic Structure - Saint Leo University Faculty
... • Proposed theory of matter (four postulates) 1. Elements are made of tiny indestructible particles called atoms. 2. Atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements. 3. Chemical combinations of elements to make different substances ...
... • Proposed theory of matter (four postulates) 1. Elements are made of tiny indestructible particles called atoms. 2. Atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements. 3. Chemical combinations of elements to make different substances ...
Introduction to Nuclear Radiation
... beam, they still continue to exist and experiments must be carefully designed to avoid confusion from scattered gamma rays. This effect is called the Compton Effect. Starting at 1 MeV and becoming increasingly important at higher energy and for high atomic number materials, are interactions of gamma ...
... beam, they still continue to exist and experiments must be carefully designed to avoid confusion from scattered gamma rays. This effect is called the Compton Effect. Starting at 1 MeV and becoming increasingly important at higher energy and for high atomic number materials, are interactions of gamma ...
Particles and Waves Summary Notes
... something called exchange particles. Each force is mediated through an exchange particle or boson. Many theories postulate the existence of a further boson, called the Higgs boson (sometimes referred to as the ‘God particle’), which isn’t involved in forces but is what gives particles mass. Attempts ...
... something called exchange particles. Each force is mediated through an exchange particle or boson. Many theories postulate the existence of a further boson, called the Higgs boson (sometimes referred to as the ‘God particle’), which isn’t involved in forces but is what gives particles mass. Attempts ...
Transfer Reaction Studies with Spectrometers
... Here, we will discuss in more details recent results on sub-barrier transfer measurement for the 40 Ca+96 Zr system in inverse kinematics [19]. In this system, the projectile (96 Zr) and target (40 Ca) are closed shell nuclei (or nearly so) for both neutrons and protons thus providing suitable condi ...
... Here, we will discuss in more details recent results on sub-barrier transfer measurement for the 40 Ca+96 Zr system in inverse kinematics [19]. In this system, the projectile (96 Zr) and target (40 Ca) are closed shell nuclei (or nearly so) for both neutrons and protons thus providing suitable condi ...
File
... 1. The reservoir stores water at a higher level than the generator below the dam, so the water has gravitational potential energy due to its higher position. 2. Water is the released into the penstock. As it flows down the penstock it loses gravitational potential energy but gains kinetic energy as ...
... 1. The reservoir stores water at a higher level than the generator below the dam, so the water has gravitational potential energy due to its higher position. 2. Water is the released into the penstock. As it flows down the penstock it loses gravitational potential energy but gains kinetic energy as ...
Chapter 1. Fundamentals of Atomic and Nuclear Physics
... The atomic mass M for a particular isotope is smaller than the sum of the individual masses of constituent particles because of the intrinsic energy associated with binding the particles (nucleons) within the nucleus IAEA Diagnostic Radiology Physics: a Handbook for Teachers and Students – chapter 1 ...
... The atomic mass M for a particular isotope is smaller than the sum of the individual masses of constituent particles because of the intrinsic energy associated with binding the particles (nucleons) within the nucleus IAEA Diagnostic Radiology Physics: a Handbook for Teachers and Students – chapter 1 ...
C. Adding acid shifts the equilibrium to the right
... are ductile. Most have extremely high melting points. Reactivity increases as you go down within a group for metals. With metals the greater the tendency to lose electrons, the more reactive the metal is. Reactive metals have low ionization energies and low electronegativities. Most nonmetals don’t ...
... are ductile. Most have extremely high melting points. Reactivity increases as you go down within a group for metals. With metals the greater the tendency to lose electrons, the more reactive the metal is. Reactive metals have low ionization energies and low electronegativities. Most nonmetals don’t ...
Nuclear Chemistry
... Nuclear Waste, continued Containment of Nuclear Waste • Nuclear waste needs to be contained so that living organisms can be shielded from radioactivity. • There are two main types of containment: on-site storage and off-site disposal. Storage of Nuclear Waste • The most common form of nuclear waste ...
... Nuclear Waste, continued Containment of Nuclear Waste • Nuclear waste needs to be contained so that living organisms can be shielded from radioactivity. • There are two main types of containment: on-site storage and off-site disposal. Storage of Nuclear Waste • The most common form of nuclear waste ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.