Introduction to Nuclear Radiation Introduction.
... Fig. 4: Counter setup for radioactivity experiments The hardware registering GM counts can be controlled either by a computer program or by the front panel. We will use the computer program first, then proceed to the front panel. First, be sure the electronics pack is on. There should be cables runn ...
... Fig. 4: Counter setup for radioactivity experiments The hardware registering GM counts can be controlled either by a computer program or by the front panel. We will use the computer program first, then proceed to the front panel. First, be sure the electronics pack is on. There should be cables runn ...
Document
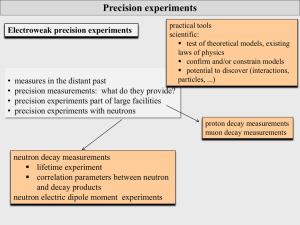
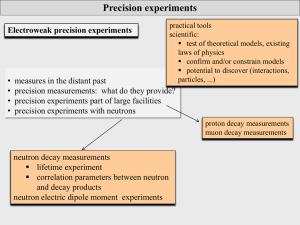
... precision experiments: particle physics Scientific precision experiments: testing the limits of our description and understanding of nature particle physics: masses and lifetimes of particles (quarks, leptons, hadrons, ...) matrix elements of transitions (CKM, PMNS, nuclear trs, ...) forces a ...
... precision experiments: particle physics Scientific precision experiments: testing the limits of our description and understanding of nature particle physics: masses and lifetimes of particles (quarks, leptons, hadrons, ...) matrix elements of transitions (CKM, PMNS, nuclear trs, ...) forces a ...
Answers
... 3) Is momentum conserved at 1? a) No, there has been a gain in momentum to the right. b) Yes, a negative particle has been produced that moves up and to the left. c) Yes, a positive particle has been produced that moves up and to the left. d) Yes, a neutral particle has been produced that moves up a ...
... 3) Is momentum conserved at 1? a) No, there has been a gain in momentum to the right. b) Yes, a negative particle has been produced that moves up and to the left. c) Yes, a positive particle has been produced that moves up and to the left. d) Yes, a neutral particle has been produced that moves up a ...
Lecture 13 - PPD - STFC Particle Physics Department
... precision experiments: particle physics Scientific precision experiments: testing the limits of our description and understanding of nature particle physics: masses and lifetimes of particles (quarks, leptons, hadrons, ...) matrix elements of transitions (CKM, PMNS, nuclear trs, ...) forces a ...
... precision experiments: particle physics Scientific precision experiments: testing the limits of our description and understanding of nature particle physics: masses and lifetimes of particles (quarks, leptons, hadrons, ...) matrix elements of transitions (CKM, PMNS, nuclear trs, ...) forces a ...
vsepr_lite_oct_2011 - chemistry11crescentsummer
... The lone pair of electrons on the central atom, N in this case, has the same spatial requirements as a pair of electrons involved in a chemical bond. This means that the overall, or electronic geometry of ammonia will also be tetrahedral. If we look only at the chemical bonds—and not at the lone pa ...
... The lone pair of electrons on the central atom, N in this case, has the same spatial requirements as a pair of electrons involved in a chemical bond. This means that the overall, or electronic geometry of ammonia will also be tetrahedral. If we look only at the chemical bonds—and not at the lone pa ...
regents chemistry midterm - irondequoit 2014_entire exam w key
... Which statement describes the type of change and the chemical properties of the product and reactants? 1) The equation represents a physical change, with the product and reactants having different chemical properties. 2) The equation represents a physical change, with the product and reactants havin ...
... Which statement describes the type of change and the chemical properties of the product and reactants? 1) The equation represents a physical change, with the product and reactants having different chemical properties. 2) The equation represents a physical change, with the product and reactants havin ...
Chapter 21. Electric Charge
... 3.Blow up a balloon and rub it against your shirt a number of times. In so doing you give the balloon a net electric charge. Now touch the balloon to the ceiling. On being released, the balloon will remain stuck to the ceiling. Why? 4. A particle is attached to a spring and is pushed so that the sp ...
... 3.Blow up a balloon and rub it against your shirt a number of times. In so doing you give the balloon a net electric charge. Now touch the balloon to the ceiling. On being released, the balloon will remain stuck to the ceiling. Why? 4. A particle is attached to a spring and is pushed so that the sp ...
ch02 lecture 7e
... Figure 2.5 Millikan’s oil-drop experiment for measuring an electron’s charge. ...
... Figure 2.5 Millikan’s oil-drop experiment for measuring an electron’s charge. ...
kovchegov
... uses a Glauber-like model for dipole amplitude with energy dependence in the exponent. figure from I. Vitev, nucl-th/0302002, see also a review by M. Gyulassy, I. Vitev, X.-N. Wang, B.-W. Zhang, nucl-th/0302077 ...
... uses a Glauber-like model for dipole amplitude with energy dependence in the exponent. figure from I. Vitev, nucl-th/0302002, see also a review by M. Gyulassy, I. Vitev, X.-N. Wang, B.-W. Zhang, nucl-th/0302077 ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.