PDF (Size: 3.8M)
... Photo-electrons are emitted spontaneously. This cannot be explained by wave theory. Free electron in a metal is emitted only when it gets certain minimum energy called “work function” ( φ ) of the metal. If the light has wave nature, free e ect on in metal may get energy gradually and some time elap ...
... Photo-electrons are emitted spontaneously. This cannot be explained by wave theory. Free electron in a metal is emitted only when it gets certain minimum energy called “work function” ( φ ) of the metal. If the light has wave nature, free e ect on in metal may get energy gradually and some time elap ...
chapter5
... Electrons only have a probability of being in a certain location, the same way the exact location of a fast moving propeller blade at any time cannot not be determined. In the quantum mechanical model, the probability of finding an electron within a certain volume of space surrounding the nucleus ca ...
... Electrons only have a probability of being in a certain location, the same way the exact location of a fast moving propeller blade at any time cannot not be determined. In the quantum mechanical model, the probability of finding an electron within a certain volume of space surrounding the nucleus ca ...
Tugas Kimia Umum
... 7. There are some exceptions to the trends of first and successive ionization energies. For each of the following pairs, explain which ionization energy would be higher: (a). IE1 of Ga or IE1 of Ge; (b). IE2 of Ga or IE2 of Ge; (c). IE3 of Ga or IE3 or Ge; (d). IE4 of Ga or IE4 of Ge Answer: (a) IE1 ...
... 7. There are some exceptions to the trends of first and successive ionization energies. For each of the following pairs, explain which ionization energy would be higher: (a). IE1 of Ga or IE1 of Ge; (b). IE2 of Ga or IE2 of Ge; (c). IE3 of Ga or IE3 or Ge; (d). IE4 of Ga or IE4 of Ge Answer: (a) IE1 ...
XII. GASEOUS ELECTRONICS Academic and Research Staff
... If we consider a gas for which the scattering is isotropic, then v = Vc2. For this case, Eqs. 17 and 18 agree very closely. For low-temperature plasmas and for v c< w, this small error in the Krook model will not be important. In a plasma for which the electron scattering cross section is not isotro ...
... If we consider a gas for which the scattering is isotropic, then v = Vc2. For this case, Eqs. 17 and 18 agree very closely. For low-temperature plasmas and for v c< w, this small error in the Krook model will not be important. In a plasma for which the electron scattering cross section is not isotro ...
SCH 3U - othsmath
... the group we go, the less strongly the valence electrons are held and the less likely the atom will pull electrons toward itself in a bond, so the lower the electronegativity value. 1) Across a period, nuclear charge increases by increments of one while each new valence electron is added. This means ...
... the group we go, the less strongly the valence electrons are held and the less likely the atom will pull electrons toward itself in a bond, so the lower the electronegativity value. 1) Across a period, nuclear charge increases by increments of one while each new valence electron is added. This means ...
recombination coefficient in a dense low-temperature plasma
... In a dense plasma, however, recombination processes involving three-body collisions become more important. These processes have been investigated by Belyaev and Budker [t] for the high temperature case kT » Ej, where Ei is the ionization energy. In the present work we compute the recombination coeff ...
... In a dense plasma, however, recombination processes involving three-body collisions become more important. These processes have been investigated by Belyaev and Budker [t] for the high temperature case kT » Ej, where Ei is the ionization energy. In the present work we compute the recombination coeff ...
Announcement Station #2 Stars Lecture 9 Basic Physics The Laws
... • No. For everyday objects, the uncertainty is still so small that it has no noticeable effect on the objects we use in everyday life. Nevertheless, the uncertainty is quantifiable and large enough to be crucial when we work with subatomic particles. • Are electrons waves or particles? • Under some ...
... • No. For everyday objects, the uncertainty is still so small that it has no noticeable effect on the objects we use in everyday life. Nevertheless, the uncertainty is quantifiable and large enough to be crucial when we work with subatomic particles. • Are electrons waves or particles? • Under some ...
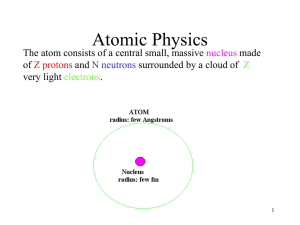
The arrangement of the subatomic particles within the atom
... y Atomic mass { This number is the mass of the nuclear particles ...
... y Atomic mass { This number is the mass of the nuclear particles ...
The Heisenberg Uncertainty Principle
... The Heisenberg Uncertainty Principle The Heisenberg uncertainty principle states that it is impossible to know both the momentum and the position of a particle at the same time. This limitation is critical when dealing with small particles such as electrons. But it does not matter for ordinary- ...
... The Heisenberg Uncertainty Principle The Heisenberg uncertainty principle states that it is impossible to know both the momentum and the position of a particle at the same time. This limitation is critical when dealing with small particles such as electrons. But it does not matter for ordinary- ...
Physics 334 Modern Physics
... based on the textbook “Modern Physics” by Thornton and Rex. I have replaced some images from the adopted text “Modern Physics” by Tipler and Llewellyn. Others images are from a variety of sources (PowerPoint clip art, Wikipedia encyclopedia etc) and were part of original lectures. Contributions are ...
... based on the textbook “Modern Physics” by Thornton and Rex. I have replaced some images from the adopted text “Modern Physics” by Tipler and Llewellyn. Others images are from a variety of sources (PowerPoint clip art, Wikipedia encyclopedia etc) and were part of original lectures. Contributions are ...
Atoms, Molecules and Ions The Early History Refer to the Chemistry
... given compound always has the same relative numbers and types of atoms 4. Chemical reactions involve reorganizations of the atoms. The atoms themselves are not changed in a chemical reaction B. Avogadro's Hypothesis 1. At the same conditions of temperature and pressure, equal volumes of different ga ...
... given compound always has the same relative numbers and types of atoms 4. Chemical reactions involve reorganizations of the atoms. The atoms themselves are not changed in a chemical reaction B. Avogadro's Hypothesis 1. At the same conditions of temperature and pressure, equal volumes of different ga ...
Chapter 9: Electrons in Atoms
... Electromagnetic radiation is a form of energy transition in which electric and magnetic fields are propagated as waves through empty space (a vacuum) or through a medium such as glass. An electric field is the region around as electrically charged particle. A magnetic field is found in the region su ...
... Electromagnetic radiation is a form of energy transition in which electric and magnetic fields are propagated as waves through empty space (a vacuum) or through a medium such as glass. An electric field is the region around as electrically charged particle. A magnetic field is found in the region su ...
Interaction of Photons with Matter
... slowly - i.e. is non-relativistic - so splitting cannot be understood.It also fails totally for atoms such as helium with two electrons where the motion of both electrons needs to be treated. ...
... slowly - i.e. is non-relativistic - so splitting cannot be understood.It also fails totally for atoms such as helium with two electrons where the motion of both electrons needs to be treated. ...
Exercises #1 - Berkeley City College
... is allowed values from 0 to (n – 1). All subshells with l = 0 and the orbitals within are designated the letter s; subshells with l = 1 are designated by letter p; subshells with l = 2 are designated letter d; subshells with l = 3 are designated letter f, and so on…To distinguish between subshells i ...
... is allowed values from 0 to (n – 1). All subshells with l = 0 and the orbitals within are designated the letter s; subshells with l = 1 are designated by letter p; subshells with l = 2 are designated letter d; subshells with l = 3 are designated letter f, and so on…To distinguish between subshells i ...
Modern Physics Review - hhs
... Radioactivity is the act of atoms breaking apart. They do this because the protons in the nucleus repel each other. Some atoms are more unstable then others because they have too many protons and not the right amount of glue (neutrons) holding them together. We can predict which isotopes are stable ...
... Radioactivity is the act of atoms breaking apart. They do this because the protons in the nucleus repel each other. Some atoms are more unstable then others because they have too many protons and not the right amount of glue (neutrons) holding them together. We can predict which isotopes are stable ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.