Light Transmission Through Randomly Rough Glass Surfaces
... In this setup, the laser was a He-Ne type with a wavelength of 633 nm and 580 uW power output. The polarizer was oriented so that the laser light is incident upon the glass surface with s-polarization (out of the page in the above diagram). We rotated the lensglass element about the center of curva ...
... In this setup, the laser was a He-Ne type with a wavelength of 633 nm and 580 uW power output. The polarizer was oriented so that the laser light is incident upon the glass surface with s-polarization (out of the page in the above diagram). We rotated the lensglass element about the center of curva ...
Atomic Structure - Hudson City School District
... Why do atoms bond? • To have filled outer electron shells! ...
... Why do atoms bond? • To have filled outer electron shells! ...
The Study of Cavitation Bubble- Surface Plasmon Resonance
... make water more colorless than gas. The role of hydrogen bonding can be determined by comparing water in its gaseous and liquid phase. When comparing the vibrational transitions between gaseous and liquid water, there is a shift to lower energy induced by hydrogen bonding. The hydrogen bonding in wa ...
... make water more colorless than gas. The role of hydrogen bonding can be determined by comparing water in its gaseous and liquid phase. When comparing the vibrational transitions between gaseous and liquid water, there is a shift to lower energy induced by hydrogen bonding. The hydrogen bonding in wa ...
The Conservation of Energy Space-Time Metric for Space Outside
... The developments of the general theory of relativity have been very good at explaining most planetary and stellar gravitational effects as well as many galactic phenomena and some inter galactic effects. There is no doubt as to the validity of the derivations of the spacetime metric of Equation (2) ...
... The developments of the general theory of relativity have been very good at explaining most planetary and stellar gravitational effects as well as many galactic phenomena and some inter galactic effects. There is no doubt as to the validity of the derivations of the spacetime metric of Equation (2) ...
oxidation number
... In general, the reactivity of nonmetals increases from left to right and decreases from top to bottom. ...
... In general, the reactivity of nonmetals increases from left to right and decreases from top to bottom. ...
Chap. 6 Conceptual Modules Fishbane
... positive work being done. Or, from the definition of work, since W = KE = KEf – KEi and we know that KEf > KEi in this case, then the work W must be positive. ...
... positive work being done. Or, from the definition of work, since W = KE = KEf – KEi and we know that KEf > KEi in this case, then the work W must be positive. ...
IPC – First Semester Exam Review Be able to classify an example
... o Noble gases are nonreactive (inert) because their valence energy level is full o Elements are generally reactive when the valence energy level is not full o Highly reactive = valence energy level is almost full, or the valence energy level is almost empty Understand how to read chemical formulas ...
... o Noble gases are nonreactive (inert) because their valence energy level is full o Elements are generally reactive when the valence energy level is not full o Highly reactive = valence energy level is almost full, or the valence energy level is almost empty Understand how to read chemical formulas ...
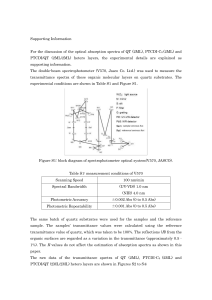
Supporting Information For the discussion of the optical absorption
... Figure S6: the UPS spectra of levels of QT (2ML), PTCDI-C8 (2ML), and QT/PTCDI-C8 (2ML/2ML). In contrast, the HOMO level is located around 5.9 eV, estimated by UPS measurements carried out by the authors [Figure S6] and Forker [18]. This result indicates that the ex-situ measurement reveals the QT ...
... Figure S6: the UPS spectra of levels of QT (2ML), PTCDI-C8 (2ML), and QT/PTCDI-C8 (2ML/2ML). In contrast, the HOMO level is located around 5.9 eV, estimated by UPS measurements carried out by the authors [Figure S6] and Forker [18]. This result indicates that the ex-situ measurement reveals the QT ...