Induction versus Conduction
... diverging rods until they are too far apart for the voltage provided by the power source. The circuit breaks and a new arc is formed at the bottom. Like a real lightning the charges jump across the separation. Notice that higher up the rods are pulled together because there they are more flexible. T ...
... diverging rods until they are too far apart for the voltage provided by the power source. The circuit breaks and a new arc is formed at the bottom. Like a real lightning the charges jump across the separation. Notice that higher up the rods are pulled together because there they are more flexible. T ...
Lecture 19: Magnetic properties and the Nephelauxetic effect
... The value of λ is negligible for very light atoms, but increases with increasing atomic weight, so that for heavier d-block elements, and for f-block elements, the orbital contribution is considerable. For 2nd and 3rd row dblock elements, λ is an order of magnitude larger than for the first-row anal ...
... The value of λ is negligible for very light atoms, but increases with increasing atomic weight, so that for heavier d-block elements, and for f-block elements, the orbital contribution is considerable. For 2nd and 3rd row dblock elements, λ is an order of magnitude larger than for the first-row anal ...
Print-ready released items - Iowa Testing Programs
... The roller coaster car’s potential energy at Position B will be closest to which of the following? A Half the roller coaster car’s potential energy at Position A CORRECT: At Position B, the roller coaster car will be almost at half the height it is at Position A. Therefore, the roller coaster car's ...
... The roller coaster car’s potential energy at Position B will be closest to which of the following? A Half the roller coaster car’s potential energy at Position A CORRECT: At Position B, the roller coaster car will be almost at half the height it is at Position A. Therefore, the roller coaster car's ...
The Role of Secondary Electrons in Low
... density was studied. The plasma density and the electron energy probability function were measured in the middle of the system. We chose two values of the SEY, i.e., 0.1 and 0.2 and we studied the influence of change of the value of the SEY on the plasma density. We studied three groups of electrons ...
... density was studied. The plasma density and the electron energy probability function were measured in the middle of the system. We chose two values of the SEY, i.e., 0.1 and 0.2 and we studied the influence of change of the value of the SEY on the plasma density. We studied three groups of electrons ...
ch-4-earth-chemistry
... Example: A neutral sodium atom has a charge of zero (equal # of protons and neutrons) and only 1 valence electron. Once it loses that valence electron, it will have 8 valence electrons and be stable and most likely, not gain or lose anymore electrons. What would be the charge on a sodium atom that l ...
... Example: A neutral sodium atom has a charge of zero (equal # of protons and neutrons) and only 1 valence electron. Once it loses that valence electron, it will have 8 valence electrons and be stable and most likely, not gain or lose anymore electrons. What would be the charge on a sodium atom that l ...
Particle accelerators and detectors
... At CERN, protons are injected into a 200m Diameter 28 GeV synchrotron ring with an energy of 50 MeV The tube is filled with protons which are injected with proton current of 100 MA for 6 uS There are 14 acceleration point spaced evenly around the ring with a potential difference between the electrod ...
... At CERN, protons are injected into a 200m Diameter 28 GeV synchrotron ring with an energy of 50 MeV The tube is filled with protons which are injected with proton current of 100 MA for 6 uS There are 14 acceleration point spaced evenly around the ring with a potential difference between the electrod ...
Laboratory: Geometrical Optics
... 38. Which material causes the largest change in direction for monochromatic red light? ...
... 38. Which material causes the largest change in direction for monochromatic red light? ...
Chapter 8 & 9 PowerPoint
... Three types of bonding • Metallic bonding – results from the attraction between metal atoms and the surrounding sea of electrons • Ionic bonding – results from the electrical attraction between positive and negative ions. • Covalent bonding – results from the sharing of electron pairs between two a ...
... Three types of bonding • Metallic bonding – results from the attraction between metal atoms and the surrounding sea of electrons • Ionic bonding – results from the electrical attraction between positive and negative ions. • Covalent bonding – results from the sharing of electron pairs between two a ...
Slide 1
... Obtain by loosing electrons. Always going to be a metal Written using the symbol with a + sign Ex. Na+ Proton – 11, electron -10 Roman numerals are used to show the charge of certain metals. • Ex: Fe 3+ is named as Iron III ...
... Obtain by loosing electrons. Always going to be a metal Written using the symbol with a + sign Ex. Na+ Proton – 11, electron -10 Roman numerals are used to show the charge of certain metals. • Ex: Fe 3+ is named as Iron III ...
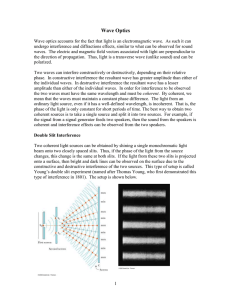
Sources of Light
... Introduction A. Background of the Study Light is the form of energy visible to the eye that is radiated by moving charged particles. Light is made of electromagnetic vibrations which have wavelengths distributed evenly between 14 to 30 millionths of an inch. ...
... Introduction A. Background of the Study Light is the form of energy visible to the eye that is radiated by moving charged particles. Light is made of electromagnetic vibrations which have wavelengths distributed evenly between 14 to 30 millionths of an inch. ...
F047063748
... and fro. Say for the 1st 5milisecond the electrons will accelerate in the positive direction of the X-axis then for the 2nd 5milisecond they will decelerate but will still keep moving in the same positive direction of the X-axis and will come to rest finally. Then when the AC will start flowing in t ...
... and fro. Say for the 1st 5milisecond the electrons will accelerate in the positive direction of the X-axis then for the 2nd 5milisecond they will decelerate but will still keep moving in the same positive direction of the X-axis and will come to rest finally. Then when the AC will start flowing in t ...
Raman Spectroscopy - Harlem Children Society
... In Raman spectroscopy, by varying the frequency of the radiation, a spectrum can be produced, showing the intensity of the exiting radiation for each frequency. This spectrum will show which frequencies of radiation have been absorbed by the molecule to raise it to higher vibrational energy states. ...
... In Raman spectroscopy, by varying the frequency of the radiation, a spectrum can be produced, showing the intensity of the exiting radiation for each frequency. This spectrum will show which frequencies of radiation have been absorbed by the molecule to raise it to higher vibrational energy states. ...