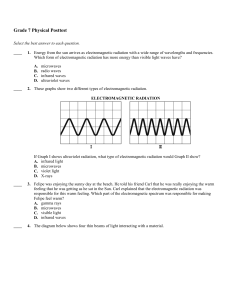
Single-photon multiple ionization processes studied by electron coincidence spectroscopy Per Linusson
... peaks in photoelectron spectra, side bands were also observed that could only be accounted for if electron-electron interactions were taken into account in the physical description in a more refined way than in the independent particle methods (see e.g. Ref. [10] and references therein). In some cas ...
... peaks in photoelectron spectra, side bands were also observed that could only be accounted for if electron-electron interactions were taken into account in the physical description in a more refined way than in the independent particle methods (see e.g. Ref. [10] and references therein). In some cas ...
Introduction to Waves
... As atoms absorb energy, electrons jump out to a higher energy level. Electrons release light when falling down to the lower energy level. Photons - bundles/packets of energy released when the electrons fall. Light: Stream of Photons ...
... As atoms absorb energy, electrons jump out to a higher energy level. Electrons release light when falling down to the lower energy level. Photons - bundles/packets of energy released when the electrons fall. Light: Stream of Photons ...
Telescopes
... • This makes it difficult to observe at near and mid-infrared wavelengths. • Because the sky is so bright at infrared wavelengths, infrared astronomers are often given what is called ``bright time’’ (when the moon is up). ``Dark time’’ is when there is little or no moon. • You can often figure out w ...
... • This makes it difficult to observe at near and mid-infrared wavelengths. • Because the sky is so bright at infrared wavelengths, infrared astronomers are often given what is called ``bright time’’ (when the moon is up). ``Dark time’’ is when there is little or no moon. • You can often figure out w ...
Chapter 6 Work and Energy continued
... force and displacement, and θ is the angle between F and s. The origin of the force does not affect the calculation of the work done. Work can be done by: gravity, elastic, friction, explosion, or human forces. ...
... force and displacement, and θ is the angle between F and s. The origin of the force does not affect the calculation of the work done. Work can be done by: gravity, elastic, friction, explosion, or human forces. ...
Measurements - Singapore A Level Notes
... compared to those located at other latitudes. Hence the total change in kinetic energy of the satellite between the Earth’s surface and in orbit will also be the lowest. Describe the concept of weight as the effect of a gravitational field on a mass. An object which placed in a gravitational field w ...
... compared to those located at other latitudes. Hence the total change in kinetic energy of the satellite between the Earth’s surface and in orbit will also be the lowest. Describe the concept of weight as the effect of a gravitational field on a mass. An object which placed in a gravitational field w ...
2-27 Potential Energy, Potential, and Work
... Problem: Two identical point charges of mass of 0.01g are placed 1m apart. The right-hand charge is released. Find its velocity when it is 10cm farther away. E Field and Force are not the same ...
... Problem: Two identical point charges of mass of 0.01g are placed 1m apart. The right-hand charge is released. Find its velocity when it is 10cm farther away. E Field and Force are not the same ...
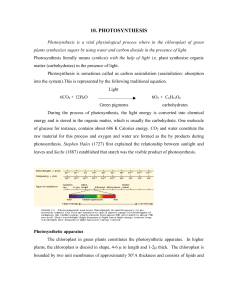
Lecture 10: Photosynthesis
... photosynthesis. Chlorophyll a molecules also absorb light energy directly. As a result of absorbing the light energy, the chlorophyll molecule gets excited. Excited states of atoms or molecules (fluorescence and phosphorescence) The normal state of the chlorophyll molecule or atom is called as groun ...
... photosynthesis. Chlorophyll a molecules also absorb light energy directly. As a result of absorbing the light energy, the chlorophyll molecule gets excited. Excited states of atoms or molecules (fluorescence and phosphorescence) The normal state of the chlorophyll molecule or atom is called as groun ...
Allowed and forbidden transitions in artificial hydrogen and helium
... circular symmetry, orbital degeneracy is lifted even at zero magnetic field, and only a twofold spin degeneracy is expected. This noncircularity does not much affect our discussion, and we still use 1s and 2p to label the orbitals for convenience (Fig. 1c). First we investigate the N ¼ 1 QD (artific ...
... circular symmetry, orbital degeneracy is lifted even at zero magnetic field, and only a twofold spin degeneracy is expected. This noncircularity does not much affect our discussion, and we still use 1s and 2p to label the orbitals for convenience (Fig. 1c). First we investigate the N ¼ 1 QD (artific ...