PPT day 3 em waves and mediums
... therefore, light waves cannot diffract very much around large obstacles, such as buildings. Thus, you cannot see around corners (but you can hear sound around corners) http://www.acoustics.salford.ac.uk/feschools/waves/diffract.htm ...
... therefore, light waves cannot diffract very much around large obstacles, such as buildings. Thus, you cannot see around corners (but you can hear sound around corners) http://www.acoustics.salford.ac.uk/feschools/waves/diffract.htm ...
Slide 1
... Potential Energy is stored energy. Energy can be stored in various forms. 1. Energy can be stored by raising an object above the ground (gravitational potential energy). 2. Energy can be stored by compressing or stretching a spring (elastic potential energy). 3. Energy can be stored in the chemical ...
... Potential Energy is stored energy. Energy can be stored in various forms. 1. Energy can be stored by raising an object above the ground (gravitational potential energy). 2. Energy can be stored by compressing or stretching a spring (elastic potential energy). 3. Energy can be stored in the chemical ...
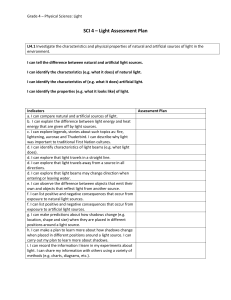
SCI 4 Light Assessment Plan
... light. I can carry out my plan to learn about the reflective properties of light. e. I can make a conclusion about the reflective properties after observing and testing. f. I can show how transparent material of different materials and shapes can change the direction of light. I can explain how this ...
... light. I can carry out my plan to learn about the reflective properties of light. e. I can make a conclusion about the reflective properties after observing and testing. f. I can show how transparent material of different materials and shapes can change the direction of light. I can explain how this ...
Solution
... 20) Which of the graphs is a plot of ln K vs. 1/T for an endothermic reaction where the change in entropy is positive? C 21) Which of the graphs is a plot of temperature versus heat added for a liquid that does not include a phase change? A 22) Which of the graphs is a plot of kinetic energy vs. fre ...
... 20) Which of the graphs is a plot of ln K vs. 1/T for an endothermic reaction where the change in entropy is positive? C 21) Which of the graphs is a plot of temperature versus heat added for a liquid that does not include a phase change? A 22) Which of the graphs is a plot of kinetic energy vs. fre ...
Chemistry Comes Alive: Part A
... 1. Solid—definite shape and volume 2. Liquid—definite volume, changeable shape 3. Gas—changeable shape and volume ...
... 1. Solid—definite shape and volume 2. Liquid—definite volume, changeable shape 3. Gas—changeable shape and volume ...
1st Semester Practice Test
... 57. What are quanta of light called? c. muons a. charms b. excitons d. photons 58. Which scientist developed the quantum mechanical model of the atom? a. Albert Einstein c. Niels Bohr b. Erwin Schrodinger d. Ernest Rutherford 59. According to the Heisenberg uncertainty principle, if th ...
... 57. What are quanta of light called? c. muons a. charms b. excitons d. photons 58. Which scientist developed the quantum mechanical model of the atom? a. Albert Einstein c. Niels Bohr b. Erwin Schrodinger d. Ernest Rutherford 59. According to the Heisenberg uncertainty principle, if th ...
DESY Summer Student Program 2011 Simulations of beam
... penetrates the surface and scatters off one or more atoms and is reflected back out, it is a “rediffused electron”. If the electron interacts inelastically with the material and releases more electrons, “true secondary electrons” are generated. In future graphics will be made allusion to this concep ...
... penetrates the surface and scatters off one or more atoms and is reflected back out, it is a “rediffused electron”. If the electron interacts inelastically with the material and releases more electrons, “true secondary electrons” are generated. In future graphics will be made allusion to this concep ...
to - Cpathshala
... The ratio of the radius of Bohr first orbit for the electron orbiting the hydrogen nucleus to that of the electron orbiting the deuterium nucleus (mass nearly twice that of H nucleus) is approximately (a) 1 : 1 (b) 1 : 2 (c) 2 : 1 (d) 1 : 4 Correct set of four quantum number for the valence (outermo ...
... The ratio of the radius of Bohr first orbit for the electron orbiting the hydrogen nucleus to that of the electron orbiting the deuterium nucleus (mass nearly twice that of H nucleus) is approximately (a) 1 : 1 (b) 1 : 2 (c) 2 : 1 (d) 1 : 4 Correct set of four quantum number for the valence (outermo ...
Astronomical Tools
... the speed of light. • The EM Spectrum includes: Radio, Microwave, Infrared, Visible, Ultraviolet, X-Rays, and Gamma Rays. • A photon, or particle of light, can be thought of as a bundle of waves that sometimes acts like a wave and sometimes like a particle. ...
... the speed of light. • The EM Spectrum includes: Radio, Microwave, Infrared, Visible, Ultraviolet, X-Rays, and Gamma Rays. • A photon, or particle of light, can be thought of as a bundle of waves that sometimes acts like a wave and sometimes like a particle. ...
2204-Unit4 – Part2 Notes
... 3. In each diagram, draw the "missing" ray (either incident or refracted) in order to appropriately show that the direction of bending is towards or away from the normal. ...
... 3. In each diagram, draw the "missing" ray (either incident or refracted) in order to appropriately show that the direction of bending is towards or away from the normal. ...
Light
... • Einstein suggested that light consisted of discrete units of energy, E = hf. Electrons could either get hit with and absorb a whole photon, or they could not. There was no in-between (getting part of a photon). • If the energy of the unit of light (photon) was not large enough to let the electron ...
... • Einstein suggested that light consisted of discrete units of energy, E = hf. Electrons could either get hit with and absorb a whole photon, or they could not. There was no in-between (getting part of a photon). • If the energy of the unit of light (photon) was not large enough to let the electron ...
Chapter 7 Solution Manual
... a) Wavelength increases from left (10–2 nm) to right (1012 nm) in Figure 7.3. The trend in increasing wavelength is: x-ray < ultraviolet < visible < infrared < microwave < radio wave. b) Frequency is inversely proportional to wavelength according to the equation c = λν, so frequency has the opposite ...
... a) Wavelength increases from left (10–2 nm) to right (1012 nm) in Figure 7.3. The trend in increasing wavelength is: x-ray < ultraviolet < visible < infrared < microwave < radio wave. b) Frequency is inversely proportional to wavelength according to the equation c = λν, so frequency has the opposite ...
path to electron - FSU High Energy Physics
... flux of the field through a surface = the total net number of field lines penetrating the surface. for a uniform field B, the flux is just the product of the field strength and the “effective” area of the surface; the effective area is the area “offered” to or “penetrated” by the field lines (i.e. t ...
... flux of the field through a surface = the total net number of field lines penetrating the surface. for a uniform field B, the flux is just the product of the field strength and the “effective” area of the surface; the effective area is the area “offered” to or “penetrated” by the field lines (i.e. t ...
The Periodic Table
... the core of an atom, called the nucleus The number of protons and neutrons add together to give the mass of the atom – each is designated a mass of 1 amu ...
... the core of an atom, called the nucleus The number of protons and neutrons add together to give the mass of the atom – each is designated a mass of 1 amu ...