Ch 8 Cellular Respiration
... Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. ...
... Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. ...
Arginine is actively transported into Neurospow
... is temperature-dependsnt with on optimum at 35’C. Omithine ...
... is temperature-dependsnt with on optimum at 35’C. Omithine ...
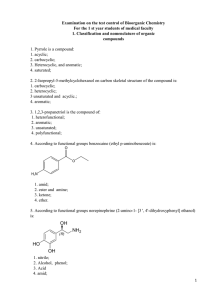
2. 2-Isopropyl-5-methylcyclohexanol on carbon skeletal
... 78. The molecule of 1-propanol present reaction centers: +1. OH acid, main, electrophilic; 2. SH-acid; 3. nucleophilic; 4. CH-acidic. 79. The molecule β-naphthol reaction centers are present: 1. OH acid; 2. SH-acid; 3. electrophilic; 4. CH-acidic. 80. Ethanethiol molecule present reaction centers: 1 ...
... 78. The molecule of 1-propanol present reaction centers: +1. OH acid, main, electrophilic; 2. SH-acid; 3. nucleophilic; 4. CH-acidic. 79. The molecule β-naphthol reaction centers are present: 1. OH acid; 2. SH-acid; 3. electrophilic; 4. CH-acidic. 80. Ethanethiol molecule present reaction centers: 1 ...
Bell Activity
... Acids taste sour, and most acids are poisonous. B. Acids Change Colors in Indicators A substance that changes color in the presence of an acid or base is an indicator. Bromthymol blue indicator ...
... Acids taste sour, and most acids are poisonous. B. Acids Change Colors in Indicators A substance that changes color in the presence of an acid or base is an indicator. Bromthymol blue indicator ...
Cell Respiration - Biology Junction
... 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NAD+; th ...
... 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NAD+; th ...
lecture2
... increasing its solubility and surface area for enzyme attack. In the acid environment of the stomach digestion of carbohydrate stops. The stomach contents (chyme) is introduced into the duodenum through the pyloric valve. The pancreatic and bile duct. open into the duodenum, their alkaline content n ...
... increasing its solubility and surface area for enzyme attack. In the acid environment of the stomach digestion of carbohydrate stops. The stomach contents (chyme) is introduced into the duodenum through the pyloric valve. The pancreatic and bile duct. open into the duodenum, their alkaline content n ...
chapter8powerpointle
... Intermediates from respiratory pathways can be used for anabolism Anabolism (build-up side of metabolism): Carbs: - Start with acetyl-CoA - Basically reverses glycolysis (but different pathway) ...
... Intermediates from respiratory pathways can be used for anabolism Anabolism (build-up side of metabolism): Carbs: - Start with acetyl-CoA - Basically reverses glycolysis (but different pathway) ...
Mader/Biology, 11/e – Chapter Outline
... 1. The electron transport chain (ETC) is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NA ...
... 1. The electron transport chain (ETC) is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. NADH and FADH2 carry the electrons to the electron transport system. 3. Members of the Chain a. NADH gives up its electrons and becomes NA ...
Amino Acid and Nucleobase Synthesis in Meteoritic Parent Bodies
... - Lowest cost amino acids (eg. G) found in most highly expressed proteins (eg. Akashi & Gojobori (2002) 3. As more amino acids added, proteins ever more useful - finally DNA/protein code takes over (eg. Wong 2005) 4. Thermodynamics + Darwinian selection may produce early codes with similar attribute ...
... - Lowest cost amino acids (eg. G) found in most highly expressed proteins (eg. Akashi & Gojobori (2002) 3. As more amino acids added, proteins ever more useful - finally DNA/protein code takes over (eg. Wong 2005) 4. Thermodynamics + Darwinian selection may produce early codes with similar attribute ...
Nitrogen Metabolism, Ammonia Degradation and Urea Formation
... Explain Negative and positive nitrogen balance. Discuss the reactions related with the degradation of amino acids. Explain ammonia formation and degradation. Discuss Urea formation. Enlist different steps of urea formation. ...
... Explain Negative and positive nitrogen balance. Discuss the reactions related with the degradation of amino acids. Explain ammonia formation and degradation. Discuss Urea formation. Enlist different steps of urea formation. ...
Plant Respiration Exchange of Gases in Plants - E
... The energy released during the electron transport system is utilised in synthesizing ATP with the help of ATP synthase (Complex V). This complex is composed of two major components, viz. F1 and F0. The F1 headpiece is a peripheral membrane protein complex. It contains the site for synthesis of ATP. ...
... The energy released during the electron transport system is utilised in synthesizing ATP with the help of ATP synthase (Complex V). This complex is composed of two major components, viz. F1 and F0. The F1 headpiece is a peripheral membrane protein complex. It contains the site for synthesis of ATP. ...
Cellular Respiration Webquest
... Each time a transfer takes place, some energy is transferred to the next molecule, but some energy is lost. ...
... Each time a transfer takes place, some energy is transferred to the next molecule, but some energy is lost. ...
Acid/Base Homeostasis - Interactive Physiology
... • When there is excess base in the body, proteins release hydrogen ion from side chains that are weak acids. • Notice that the shape of the protein did not change much here because only small amounts of acid or base were added. If the pH increases or decreases too much, the proteins may become denat ...
... • When there is excess base in the body, proteins release hydrogen ion from side chains that are weak acids. • Notice that the shape of the protein did not change much here because only small amounts of acid or base were added. If the pH increases or decreases too much, the proteins may become denat ...
milk-spoilage-biochemical-activities-of-microbes
... Micrococcus spp.: – these are very notorious and may even cause the proteolysis of freshly drawn milk as some of them inhabit the cow’s udder Streptococcus faecalis and Streptococcus liquifasciens: – very active proteolytic bacteria that may cause proteolysis of pasteurized milk Spores of some strai ...
... Micrococcus spp.: – these are very notorious and may even cause the proteolysis of freshly drawn milk as some of them inhabit the cow’s udder Streptococcus faecalis and Streptococcus liquifasciens: – very active proteolytic bacteria that may cause proteolysis of pasteurized milk Spores of some strai ...
cheese - Genootschap Melkkunde
... - Carboxypeptidase catalyses the removal of one or two amino acid residues from the C-terminus of the protein. - Aminopeptidase catalyses the removal of one or two single amino acid residue from the N-terminus of the protein. ...
... - Carboxypeptidase catalyses the removal of one or two amino acid residues from the C-terminus of the protein. - Aminopeptidase catalyses the removal of one or two single amino acid residue from the N-terminus of the protein. ...
Cellular Respiration - Science with Ms. Wood!
... The summary equation of cellular respiration. The difference between fermentation and cellular respiration. The role of glycolysis in oxidizing glucose to two molecules of pyruvate The process that brings pyruvate from the cytosol into the mitochondria and introduces it into the citric acid cyc ...
... The summary equation of cellular respiration. The difference between fermentation and cellular respiration. The role of glycolysis in oxidizing glucose to two molecules of pyruvate The process that brings pyruvate from the cytosol into the mitochondria and introduces it into the citric acid cyc ...
Lecture 40
... Biosynthetic pathways found in plants are responsible for the production of amino acids that are "essential" to humans. (Similar pathways are found in bacteria). Where does this come from? ...
... Biosynthetic pathways found in plants are responsible for the production of amino acids that are "essential" to humans. (Similar pathways are found in bacteria). Where does this come from? ...
Chapter 8 - South Sevier High School
... a. NADH gives up its electrons and becomes NAD+; the next carrier then gains electrons and is thereby reduced. b. At each sequential redox reaction, energy is released to form ATP molecules. c. Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in th ...
... a. NADH gives up its electrons and becomes NAD+; the next carrier then gains electrons and is thereby reduced. b. At each sequential redox reaction, energy is released to form ATP molecules. c. Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in th ...
Feodor Lynen - Nobel Lecture
... It was possible to assume with fair certainty from these results that the succinic acid produced by yeast from acetate is formed via citric acid7. Sonderhoff’s experiments with deuterated acetic acid led to another important discovery. In the analysis of the yeast cells themselves, it was found that ...
... It was possible to assume with fair certainty from these results that the succinic acid produced by yeast from acetate is formed via citric acid7. Sonderhoff’s experiments with deuterated acetic acid led to another important discovery. In the analysis of the yeast cells themselves, it was found that ...
Identification of α-amino acids by hydrophilic interaction
... ing plant growth is a well-known method to increase crop yield and quality, beside that amino acids may form che lates with trace elements and rear earth minerals which can kill bacteria and insects and decrease an amount of residual pesticides [2]. Usually amino acid based fertilizers are recommen ...
... ing plant growth is a well-known method to increase crop yield and quality, beside that amino acids may form che lates with trace elements and rear earth minerals which can kill bacteria and insects and decrease an amount of residual pesticides [2]. Usually amino acid based fertilizers are recommen ...
The Effect of Protein Loads on Plasma Amino Acid Levels
... (Felig, 1973). The branched-chain amino acids are deaminated more slowly than other amino acids in the liver, and normally depend on the extrahepatic tissues for their metabolism (Miller, 1962; Ning, Lowenstein & Davidson, 1967; Felig, 1973). This explains the finding that they show the most sustain ...
... (Felig, 1973). The branched-chain amino acids are deaminated more slowly than other amino acids in the liver, and normally depend on the extrahepatic tissues for their metabolism (Miller, 1962; Ning, Lowenstein & Davidson, 1967; Felig, 1973). This explains the finding that they show the most sustain ...
Influence of free linoleic acid on the fatty acids profile of fermentation
... conjugation of cis-9, cis-12 double bonds was identified as linoleic acid isomerase (EC 5.3.1.5). It is a particulate enzyme bound to the bacterial cell membrane [13] and demonstrates an absolute substrate requirement for a cis-9, cis-12 diene system and a free carboxyl group [14]. Biohydrogenation ...
... conjugation of cis-9, cis-12 double bonds was identified as linoleic acid isomerase (EC 5.3.1.5). It is a particulate enzyme bound to the bacterial cell membrane [13] and demonstrates an absolute substrate requirement for a cis-9, cis-12 diene system and a free carboxyl group [14]. Biohydrogenation ...
MS Word Version - Interactive Physiology
... • When there is excess base in the body, proteins release hydrogen ion from side chains that are weak acids. • Notice that the shape of the protein did not change much here because only small amounts of acid or base were added. If the pH increases or decreases too much, the proteins may become denat ...
... • When there is excess base in the body, proteins release hydrogen ion from side chains that are weak acids. • Notice that the shape of the protein did not change much here because only small amounts of acid or base were added. If the pH increases or decreases too much, the proteins may become denat ...
MS Word Version
... • When there is excess base in the body, proteins release hydrogen ion from side chains that are weak acids. • Notice that the shape of the protein did not change much here because only small amounts of acid or base were added. If the pH increases or decreases too much, the proteins may become denat ...
... • When there is excess base in the body, proteins release hydrogen ion from side chains that are weak acids. • Notice that the shape of the protein did not change much here because only small amounts of acid or base were added. If the pH increases or decreases too much, the proteins may become denat ...
Butyric acid
Butyric acid (from Greek βούτῡρον, meaning ""butter""), also known under the systematic name butanoic acid, abbreviated BTA, is a carboxylic acid with the structural formula CH3CH2CH2-COOH. Salts and esters of butyric acid are known as butyrates or butanoates. Butyric acid is found in milk, especially goat, sheep and buffalo milk, butter, parmesan cheese, and as a product of anaerobic fermentation (including in the colon and as body odor). It has an unpleasant smell and acrid taste, with a sweetish aftertaste (similar to ether). It can be detected by mammals with good scent detection abilities (such as dogs) at 10 parts per billion, whereas humans can detect it in concentrations above 10 parts per million.Butyric acid is present in, and is the main distinctive smell of, human vomit.Butyric acid was first observed (in impure form) in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. The name of butyric acid comes from the Latin word for butter, butyrum (or buturum), the substance in which butyric acid was first found.