Molecular Modeling of Hydrophobic Organic Contaminants
... Modeling Humic Acids and Asphaltenes • There are two major impediments to this conventional approach. • First, the structure elucidation process is carried out manually • This may be prohibitively time consuming for multifunctional geomacromolecules such as humic acids and asphaltenes. ...
... Modeling Humic Acids and Asphaltenes • There are two major impediments to this conventional approach. • First, the structure elucidation process is carried out manually • This may be prohibitively time consuming for multifunctional geomacromolecules such as humic acids and asphaltenes. ...
Supplementary Information
... We have analyzed the catalytic effect of six meteorites of the carbonaceous chondrite type, namely Allan Hills 84028 (ALH 84028, class CV3), Elephant Moraine 92042 (EET 92042, class CR2), Miller Range 05024 (MIL 05024, class CO3), Larkman Nunatak 04318 (LAR 04318, CK4), Grosvenor Mountains 95551 (GR ...
... We have analyzed the catalytic effect of six meteorites of the carbonaceous chondrite type, namely Allan Hills 84028 (ALH 84028, class CV3), Elephant Moraine 92042 (EET 92042, class CR2), Miller Range 05024 (MIL 05024, class CO3), Larkman Nunatak 04318 (LAR 04318, CK4), Grosvenor Mountains 95551 (GR ...
File - Fidaa`s Level 2 Portfolio
... with like mad chemicals. We should get the organic apples instead, I hear they don’t have chemicals.” Write a short paragraph in response to your sister. Your paragraph should demonstrate a strong understanding of what "organic" means when speaking about agriculture, as well as the difference betwee ...
... with like mad chemicals. We should get the organic apples instead, I hear they don’t have chemicals.” Write a short paragraph in response to your sister. Your paragraph should demonstrate a strong understanding of what "organic" means when speaking about agriculture, as well as the difference betwee ...
Acids and Bases
... source are placed in a beaker of water, the light bulb will not glow. If an electrolyte, such as sodium chloride, is dissolved in the water, the light bulb will glow because the solution can now conduct electricity. The amount of electric current that can be carried by an electrolyte solution is pro ...
... source are placed in a beaker of water, the light bulb will not glow. If an electrolyte, such as sodium chloride, is dissolved in the water, the light bulb will glow because the solution can now conduct electricity. The amount of electric current that can be carried by an electrolyte solution is pro ...
Sulfuric Acid
... pigment production, steel pickling, nonferrous metals extraction, and the manufacture of explosives, detergents, plastics, and man-made fibers. Many specialty areas of the chemical industry also use varying amounts of sulfuric acid including the production of dyes, pharmaceuticals, and fluorine chem ...
... pigment production, steel pickling, nonferrous metals extraction, and the manufacture of explosives, detergents, plastics, and man-made fibers. Many specialty areas of the chemical industry also use varying amounts of sulfuric acid including the production of dyes, pharmaceuticals, and fluorine chem ...
acids - WordPress.com
... 7. The residual liquid can be filtered away and the crystals can be carefully collected and rinsed & dried between 2 pieces of filter paper. ...
... 7. The residual liquid can be filtered away and the crystals can be carefully collected and rinsed & dried between 2 pieces of filter paper. ...
Safety Data Sheet - Fisher Scientific
... SECTION 4 : First aid measures Description of first aid measures After inhalation: Move exposed individual to fresh air. Loosen clothing as necessary and position individual in a comfortable position.Provide oxygen if breathing is difficult. Seek immediate medical advice. After skin contact: Rinse t ...
... SECTION 4 : First aid measures Description of first aid measures After inhalation: Move exposed individual to fresh air. Loosen clothing as necessary and position individual in a comfortable position.Provide oxygen if breathing is difficult. Seek immediate medical advice. After skin contact: Rinse t ...
Acid K a
... NO3- - worthless, NH4+ - weak acid, acidic K+ - worthless, I- - worthless, neutral Li+ - worthless, C2H3O2- - weak base, basic Cl- - worthless, C6H5NH3+ - weak acid, acidic K+ - worthless, F- - weak base, basic ...
... NO3- - worthless, NH4+ - weak acid, acidic K+ - worthless, I- - worthless, neutral Li+ - worthless, C2H3O2- - weak base, basic Cl- - worthless, C6H5NH3+ - weak acid, acidic K+ - worthless, F- - weak base, basic ...
Diazotization-Coupling Reaction--
... 6. After all of the sulfanilic acid dissolves completely, remove the Erlenmeyer flask and allow it to cool to room temperature on the bench top. 7. Weigh 0.08-g sodium nitrite, NaNO2, and transfer it to the cooled Erlenmeyer flask; stir the solution until the solid dissolves. 8. Cool the 25-mL Erlen ...
... 6. After all of the sulfanilic acid dissolves completely, remove the Erlenmeyer flask and allow it to cool to room temperature on the bench top. 7. Weigh 0.08-g sodium nitrite, NaNO2, and transfer it to the cooled Erlenmeyer flask; stir the solution until the solid dissolves. 8. Cool the 25-mL Erlen ...
A NOVEL BIOCHEMICAL METHOD FOR PRODUCTION OF AN ANTIBACTERIAL DRUG
... A solution of guanidine hydrochloride (12 g, 0.126 mol) in methanol was neutralized by a methanolic solution of of sodium (3 g, 0.130 mol). The mixture of benzal nitriles (10.4 g, 0.04 mol) was dissolved in the guanidine solution, and the resulting solution was ...
... A solution of guanidine hydrochloride (12 g, 0.126 mol) in methanol was neutralized by a methanolic solution of of sodium (3 g, 0.130 mol). The mixture of benzal nitriles (10.4 g, 0.04 mol) was dissolved in the guanidine solution, and the resulting solution was ...
Lab 13
... 6. After all of the sulfanilic acid dissolves completely, remove the Erlenmeyer flask and allow it to cool to room temperature on the bench top. 7. Weigh 0.08-g sodium nitrite, NaNO2, and transfer it to the cooled Erlenmeyer flask; stir the solution until the solid dissolves. 8. Cool the 25-mL Erlen ...
... 6. After all of the sulfanilic acid dissolves completely, remove the Erlenmeyer flask and allow it to cool to room temperature on the bench top. 7. Weigh 0.08-g sodium nitrite, NaNO2, and transfer it to the cooled Erlenmeyer flask; stir the solution until the solid dissolves. 8. Cool the 25-mL Erlen ...
Acid Base PPT - mvhs
... because an Arrhenius acid increases H+ concentration, when dissolved in water. An Arrhenius base solution contains an excess of OH- ions because it increases OH- concentration, when dissolved in ...
... because an Arrhenius acid increases H+ concentration, when dissolved in water. An Arrhenius base solution contains an excess of OH- ions because it increases OH- concentration, when dissolved in ...
SATL-POC - Systematic Approach to Teaching
... units. The absorption of O-H stretching appears as a broad band near 3000 cm-1. The νC=O stretching absorption in aliphatic acids occurs at 1725-1700 cm-1. • Some of the acids viz., acetic acid, benzoic acid, exist as dimmers due to hydrogen bonding. Formation of bridge lowers the force constants an ...
... units. The absorption of O-H stretching appears as a broad band near 3000 cm-1. The νC=O stretching absorption in aliphatic acids occurs at 1725-1700 cm-1. • Some of the acids viz., acetic acid, benzoic acid, exist as dimmers due to hydrogen bonding. Formation of bridge lowers the force constants an ...
Carboxylic Acid Derivatives and Nitrogen Cpds
... H decolourises aqueous bromine with the formation of a white precipitate K. No orange crystals are observed when 2,4-dinitrophenyihydrazine is added to H. However when H is heated with alkaline aqueous iodine and then followed by careful acidification, some yellow crystals are produced together with ...
... H decolourises aqueous bromine with the formation of a white precipitate K. No orange crystals are observed when 2,4-dinitrophenyihydrazine is added to H. However when H is heated with alkaline aqueous iodine and then followed by careful acidification, some yellow crystals are produced together with ...
Part1. Acid rain formation. 1. Discovery of acid rain.
... Acid rain today is produced mainly from emissions of sulfur oxides (SOx) and nitrogen oxides (NOx) generated by human activities, which are oxidized in the atmosphere to form sulfuric and nitric acids, respectively. However, various species can be found in rainwater. ...
... Acid rain today is produced mainly from emissions of sulfur oxides (SOx) and nitrogen oxides (NOx) generated by human activities, which are oxidized in the atmosphere to form sulfuric and nitric acids, respectively. However, various species can be found in rainwater. ...
Chemistry -- Acids and Bases
... H2O(l) + NH3(aq) → NH4+(aq) + OHWhich is the acid and which is the base? Water can be both an acid or a base – depending on what it reacts with Amphiprotic compounds: Compounds that can act as either an acid or a base ...
... H2O(l) + NH3(aq) → NH4+(aq) + OHWhich is the acid and which is the base? Water can be both an acid or a base – depending on what it reacts with Amphiprotic compounds: Compounds that can act as either an acid or a base ...
Teacher`s Guide
... Medicine dropper bottles can also be used to make and store oleic acid solutions. ...
... Medicine dropper bottles can also be used to make and store oleic acid solutions. ...
4 Acid Base Solutions
... a. Which is the strongest among the four acids? Justify your answer quantitatively. Trichloroacetic acid (CCl3COOH) is the strongest acid because it has the most negative pKa (which is the largest Ka). b. Provide a reason using the molecular structure to explain why the acid in part a. is the strong ...
... a. Which is the strongest among the four acids? Justify your answer quantitatively. Trichloroacetic acid (CCl3COOH) is the strongest acid because it has the most negative pKa (which is the largest Ka). b. Provide a reason using the molecular structure to explain why the acid in part a. is the strong ...
Lecture notes Chapters 10
... Naming of carboxylic acids: IUPAC names for carboxylic acids are similar to alcohols except the final “-e” of the parent alkane is dropped and replaced by “-oic acid”. The parent chain should contain the carboxyl group (-COOH). Number the chain beginning with the carbon of the carboxyl group. Becaus ...
... Naming of carboxylic acids: IUPAC names for carboxylic acids are similar to alcohols except the final “-e” of the parent alkane is dropped and replaced by “-oic acid”. The parent chain should contain the carboxyl group (-COOH). Number the chain beginning with the carbon of the carboxyl group. Becaus ...
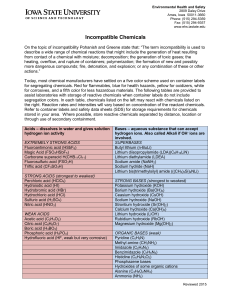
Incompatible Chemicals
... heating, overflow, and rupture of containers; polymerization; the formation of new and possibly more dangerous compounds; fire, detonation, and explosion; or any combination of these or other actions.” Today, most chemical manufacturers have settled on a five color scheme used on container labels fo ...
... heating, overflow, and rupture of containers; polymerization; the formation of new and possibly more dangerous compounds; fire, detonation, and explosion; or any combination of these or other actions.” Today, most chemical manufacturers have settled on a five color scheme used on container labels fo ...
acids and bases - Althea`s Academy
... A variation of the colorimetric method which is more practical includes strips of paper impregnated with an indicator ◦ Merely dipping the paper in the unknown sol’n produces a color on the paper which can be compared with charts supplied by the manufacture ◦ This is of value to diabetic patients fo ...
... A variation of the colorimetric method which is more practical includes strips of paper impregnated with an indicator ◦ Merely dipping the paper in the unknown sol’n produces a color on the paper which can be compared with charts supplied by the manufacture ◦ This is of value to diabetic patients fo ...
IE EA
... f) SF6 Neither; the coordination number of six is rarely exceeded so that this molecule does not act as a Lewis acid and the high electronegativity of fluorine does not allow for it to act as a base. g) PCl5 Acidic; this compound reacts with a wide variety of Lewis bases to form adducts. h) (CH3)3N ...
... f) SF6 Neither; the coordination number of six is rarely exceeded so that this molecule does not act as a Lewis acid and the high electronegativity of fluorine does not allow for it to act as a base. g) PCl5 Acidic; this compound reacts with a wide variety of Lewis bases to form adducts. h) (CH3)3N ...
Acid throwing

Acid throwing, also called an acid attack, a vitriol attack or vitriolage, is a form of violent assault defined as the act of throwing acid or a similarly corrosive substance onto the body of another ""with the intention to disfigure, maim, torture, or kill."" Perpetrators of these attacks throw acid at their victims, usually at their faces, burning them, and damaging skin tissue, often exposing and sometimes dissolving the bones. The most common types of acid used in these attacks are sulfuric and nitric acid. Hydrochloric acid is sometimes used, but is much less damaging. The long term consequences of these attacks may include blindness, as well as permanent scarring of the face and body, along with far-reaching social, psychological, and economic difficulties.Today, acid attacks are reported in many parts of the world. Since the 1990s, Bangladesh has been reporting the highest number of attacks and highest incidence rates for women, with 3,512 Bangladeshi people acid attacked between 1999 and 2013. Although acid attacks occur all over the world, including in Europe and the United States, this type of violence is mainly concentrated in South Asia.