Answers to Coursebook questions – Chapter J1
... no quantum numbers other than energy, and so the only quantum number that can separate two electrons is the spin. One electron can have spin up and the other spin down. So we can have at most two electrons. In the other shells we can have more electrons because the state has other quantum numbers su ...
... no quantum numbers other than energy, and so the only quantum number that can separate two electrons is the spin. One electron can have spin up and the other spin down. So we can have at most two electrons. In the other shells we can have more electrons because the state has other quantum numbers su ...
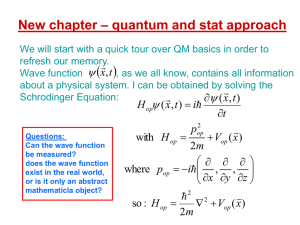
Quantum mechanics is the theory that we use to describe the
... and more prominent. The Heisenberg uncertainty principle gives us a limit as to how much we can know about a system. The more we try to pin down space and time, the greater the indeterminancy in the system grows, in that the values a system can take grow larger and larger – the values can fluctuate ...
... and more prominent. The Heisenberg uncertainty principle gives us a limit as to how much we can know about a system. The more we try to pin down space and time, the greater the indeterminancy in the system grows, in that the values a system can take grow larger and larger – the values can fluctuate ...
lecture 7
... • Now there is a difference from the particlein-a-box problem: here there is a potential energy involved …. • Coulombic attraction between the proton and electron • V = Ze2 / r ...
... • Now there is a difference from the particlein-a-box problem: here there is a potential energy involved …. • Coulombic attraction between the proton and electron • V = Ze2 / r ...
DirectProducts
... Calculations will include both p2 and not distinguish the contributions from either case. Two electrons (in momentum states p1 and p2) enter… ...
... Calculations will include both p2 and not distinguish the contributions from either case. Two electrons (in momentum states p1 and p2) enter… ...
3.2 Conserved Properties/Constants of Motion
... These quantum numbers are a adequate description of an electronic state of an Hydrogen atom (But who can for example imagine the Eigenvector of the rotational momentum operator?). These information allow to calculate the atomic orbitals. BUT: the electron is not somewhere in this orbital with a well ...
... These quantum numbers are a adequate description of an electronic state of an Hydrogen atom (But who can for example imagine the Eigenvector of the rotational momentum operator?). These information allow to calculate the atomic orbitals. BUT: the electron is not somewhere in this orbital with a well ...
Tutorial 1 - NUS Physics
... Express this state in the energy representation. Write down the energy operator in each of these three representations. Calculate the expectation value of the energy. Do this calculation three times, once in each of the representations. f) Using whichever representation you like best, find the rms d ...
... Express this state in the energy representation. Write down the energy operator in each of these three representations. Calculate the expectation value of the energy. Do this calculation three times, once in each of the representations. f) Using whichever representation you like best, find the rms d ...
Quantum mechanics is the theory that we use to describe the
... Spin angular momentum in quantum mechanics does not arise from a particle actually spinning like a top, rather it is an intrinsic property of a particle, like its mass. An important thing to note is that spin is quantised. It can only have discrete values. For example, protons, neutrons and electron ...
... Spin angular momentum in quantum mechanics does not arise from a particle actually spinning like a top, rather it is an intrinsic property of a particle, like its mass. An important thing to note is that spin is quantised. It can only have discrete values. For example, protons, neutrons and electron ...
29 jul 2016 classical monatomic ideal gas . L10–1 Classical
... well-known Gaussian integral −∞ dx e−ax = π/a. Classical Monatomic Ideal Gas Thermodynamics • Setup: As a first example of application of the equipartition principle, consider a monatomic ideal gas. The Hamiltonian is a sum of terms from individual non-interacting particles; if the particles are sin ...
... well-known Gaussian integral −∞ dx e−ax = π/a. Classical Monatomic Ideal Gas Thermodynamics • Setup: As a first example of application of the equipartition principle, consider a monatomic ideal gas. The Hamiltonian is a sum of terms from individual non-interacting particles; if the particles are sin ...
Chern-Simons theory and the fractional quantum Hall effect
... and effectively each quantum number is increased by unit: mi → mi + 1, as can be verified just expanding the Laughlin function. Each electron jumps from a state with a given angular momentum to another (from a circular orbit to another with bigger radius if you want), leaving an empty state with m = ...
... and effectively each quantum number is increased by unit: mi → mi + 1, as can be verified just expanding the Laughlin function. Each electron jumps from a state with a given angular momentum to another (from a circular orbit to another with bigger radius if you want), leaving an empty state with m = ...
Dyson Equation and Self-Consistent Green`s
... system with one or more particle cannot be extracted from this approximation. These observations become immediately clear when one realizes that the diagonal elements of G(1) have a double pole at the sp energy corresponding to the one of G(0) whereas the exact sp propagator has simple poles (at di ...
... system with one or more particle cannot be extracted from this approximation. These observations become immediately clear when one realizes that the diagonal elements of G(1) have a double pole at the sp energy corresponding to the one of G(0) whereas the exact sp propagator has simple poles (at di ...
The Scattering Green`s Function: Getting the Signs Straight
... It is worth taking a little time to discuss the statement that G+ (x, x0 ) is the Helmholtz Equation Green’s Function, namely (6.2.12), and use this to verify the overall minus sign. Take k = 0, in which case G+ (x, x0 ) is just −1/4π times the electrostatic potential at x for a unit charge located ...
... It is worth taking a little time to discuss the statement that G+ (x, x0 ) is the Helmholtz Equation Green’s Function, namely (6.2.12), and use this to verify the overall minus sign. Take k = 0, in which case G+ (x, x0 ) is just −1/4π times the electrostatic potential at x for a unit charge located ...
REVIEW OF WAVE MECHANICS
... Of course if the initial wave function is not one the eigenfunctions, the product of uncertainties will be greater than zero. ...
... Of course if the initial wave function is not one the eigenfunctions, the product of uncertainties will be greater than zero. ...
Correlation Functions and Diagrams
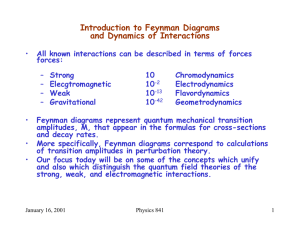
... formulation. They contain the physical information we are interested in (e.g. scattering amplitudes) and have a simple expansion in terms of Feynman diagrams. This chapter develops this formalism, which will be the language used for the rest of the course. 1 Sources The path integral gives us the ti ...
... formulation. They contain the physical information we are interested in (e.g. scattering amplitudes) and have a simple expansion in terms of Feynman diagrams. This chapter develops this formalism, which will be the language used for the rest of the course. 1 Sources The path integral gives us the ti ...
The Impact of Special Relativity in Nuclear Physics: It`s not just E=Mc 2
... The equation for E gives us two possible approaches to make a relativistic quantum mechanics. Call Y the wave function: ...
... The equation for E gives us two possible approaches to make a relativistic quantum mechanics. Call Y the wave function: ...