Beginning Chemistry
... its structure, the changes which it undergoes, and the laws governing those changes. Matter is anything that has mass and occupies space. Any material object, no matter how large or small, is composed of matter. In contrast, light, heat, and sound are forms of energy. Energy is the ability to produc ...
... its structure, the changes which it undergoes, and the laws governing those changes. Matter is anything that has mass and occupies space. Any material object, no matter how large or small, is composed of matter. In contrast, light, heat, and sound are forms of energy. Energy is the ability to produc ...
A New Form of Matter (pdf, 217 kB)
... magnetic traps, the atoms overlapped and formed a single giant (by atomic standards) matter wave. Says Ketterle: "Pictures of BECs can be regarded as photographs of wave functions" -- that is, solutions to Schrodinger's equation. Working independently in 1995, Eric Cornell (National Institute of Sta ...
... magnetic traps, the atoms overlapped and formed a single giant (by atomic standards) matter wave. Says Ketterle: "Pictures of BECs can be regarded as photographs of wave functions" -- that is, solutions to Schrodinger's equation. Working independently in 1995, Eric Cornell (National Institute of Sta ...
Chem Curr - New Haven Science
... Chemistry is a study of the fundamental structure of matter that serves as a basic understanding of science needed in today’s world. It is a study of matter, energy, atomic and molecular structure, composition, bonding, the periodic law, chemical equations, acid-base reactions, solutions, gas laws, ...
... Chemistry is a study of the fundamental structure of matter that serves as a basic understanding of science needed in today’s world. It is a study of matter, energy, atomic and molecular structure, composition, bonding, the periodic law, chemical equations, acid-base reactions, solutions, gas laws, ...
Practice Exam II
... Pb(NO3)2 + K2CrO4 PbCrO4 + 2KNO3 ? A)Pb(NO3)2/K2CrO4 or Pb(NO3)2/PbCrO4 B)K2CrO4/PbCrO4 or K2CrO4/2KNO3 C)Pb(NO3)2/2KNO3 or 2Pb(NO3)2/2K2CrO4 D) 3K2CrO4/3PbCrO4 or 2Pb(NO3)2/4KNO3 E) All of the above are correct unit-conversion factors. Note: p. p. 112-113: example & problem 4.16. Note that if the ...
... Pb(NO3)2 + K2CrO4 PbCrO4 + 2KNO3 ? A)Pb(NO3)2/K2CrO4 or Pb(NO3)2/PbCrO4 B)K2CrO4/PbCrO4 or K2CrO4/2KNO3 C)Pb(NO3)2/2KNO3 or 2Pb(NO3)2/2K2CrO4 D) 3K2CrO4/3PbCrO4 or 2Pb(NO3)2/4KNO3 E) All of the above are correct unit-conversion factors. Note: p. p. 112-113: example & problem 4.16. Note that if the ...
On the Convergence of Atomic Charges with the Size of the
... Atomic charges are an important tool to study electronic structure and chemical reactivity in, for example, protein reaction mechanisms. The following examples illustrate their importance: molecular force fields use charges to model electrostatic interactions1; the equilibrium constant of acid disso ...
... Atomic charges are an important tool to study electronic structure and chemical reactivity in, for example, protein reaction mechanisms. The following examples illustrate their importance: molecular force fields use charges to model electrostatic interactions1; the equilibrium constant of acid disso ...
Review Sheet on Determining Term Symbols
... STEP 2 (Values of ML and MS) For a d orbital, ml may range from +2 through –2 so there are 5 acceptable ml values. The maximum value of ML for 2 electrons is when m1 = +2 and when m2 = +2 or ML = 4 (this can only occur if MS = 0. Therefore, ML will range +4, +3, …, -4. The allowed values for MS are ...
... STEP 2 (Values of ML and MS) For a d orbital, ml may range from +2 through –2 so there are 5 acceptable ml values. The maximum value of ML for 2 electrons is when m1 = +2 and when m2 = +2 or ML = 4 (this can only occur if MS = 0. Therefore, ML will range +4, +3, …, -4. The allowed values for MS are ...
BTEC National in Applied Science Unit 01 Sample redacted web
... You should already know about the structure of an atom. The nucleus contains positive protons and neutral nutrons. Surrounding the nucleus are energy shells containing negative electrons. You should also know that protons and neutrons both have a relative mass of 1 and that the relative mass of an e ...
... You should already know about the structure of an atom. The nucleus contains positive protons and neutral nutrons. Surrounding the nucleus are energy shells containing negative electrons. You should also know that protons and neutrons both have a relative mass of 1 and that the relative mass of an e ...
экзаменационные тесты по органической химии
... 31. Which particle in the atom has a neutral charge. a. proton b. neutron c. electron d. nucleus 32. Atoms become ions by losing or gaining which particle? a. neutron b. ion c. electron d. proton 33. If a neutral atom has 10 protons and 10 electrons, what charge will the resulting ion have if the at ...
... 31. Which particle in the atom has a neutral charge. a. proton b. neutron c. electron d. nucleus 32. Atoms become ions by losing or gaining which particle? a. neutron b. ion c. electron d. proton 33. If a neutral atom has 10 protons and 10 electrons, what charge will the resulting ion have if the at ...
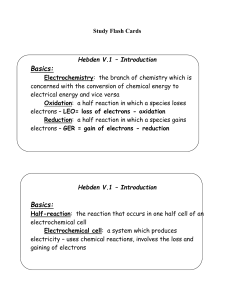
Hebden V.2 – Oxidation Numbers
... Oxidation numbers: charge an atom would have if the species containing the atom where made up of ions The sum of all positive charges and negative charges must equal the overall charge on the species Step 1: write the formula for the molecule Step 2: write the known oxidation numbers or charges belo ...
... Oxidation numbers: charge an atom would have if the species containing the atom where made up of ions The sum of all positive charges and negative charges must equal the overall charge on the species Step 1: write the formula for the molecule Step 2: write the known oxidation numbers or charges belo ...
Three-dimensional Child–Langmuir law for uniform hot electron
... electron emission in planar and cylindrical gap, including the effects of finite emission energy. It is found that the enhancement of 3D CL law 共in terms of 1D CL law兲 can be written in a general form of JC关3D兴 / JC关1D兴 = 1 + F ⫻ G, where F is the normalized mean position of 1D electron flow in clas ...
... electron emission in planar and cylindrical gap, including the effects of finite emission energy. It is found that the enhancement of 3D CL law 共in terms of 1D CL law兲 can be written in a general form of JC关3D兴 / JC关1D兴 = 1 + F ⫻ G, where F is the normalized mean position of 1D electron flow in clas ...
TEKS Presentation Properties of Matter
... Characteristics of a substance that are observed when it reacts (changes) to produce one or more different substances. Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chem ...
... Characteristics of a substance that are observed when it reacts (changes) to produce one or more different substances. Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chem ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.