Ionization methods - 2-CI - Florida International University
... molecules, hydrogen transfer may not occur and instead the secondary ions react with molecules (M) to give adduct ions, rather than protonated molecules, ...
... molecules, hydrogen transfer may not occur and instead the secondary ions react with molecules (M) to give adduct ions, rather than protonated molecules, ...
chapt 2
... Creating different chemical substances by forming and breaking chemical bonds. Remember: Atoms form chemical bonds to fill their outermost electron energy levels, achieving stability. ...
... Creating different chemical substances by forming and breaking chemical bonds. Remember: Atoms form chemical bonds to fill their outermost electron energy levels, achieving stability. ...
Sodium D Line Data
... The sodium polarizabilities are tabulated in Table 6. Notice that the differences in the excited state and ground state scalar polarizabilities are given, rather than the excited state polarizabilities, since these are the quantities that were actually measured experimentally. The polarizabilities gi ...
... The sodium polarizabilities are tabulated in Table 6. Notice that the differences in the excited state and ground state scalar polarizabilities are given, rather than the excited state polarizabilities, since these are the quantities that were actually measured experimentally. The polarizabilities gi ...
chem1a_ch02_lecture - Santa Rosa Junior College
... the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons to have the same number of electrons as 10Ne. The ion is Al3+ ...
... the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons to have the same number of electrons as 10Ne. The ion is Al3+ ...
chem1a_ch02_lecture - Santa Rosa Junior College
... the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons to have the same number of electrons as 10Ne. The ion is Al3+ ...
... the same number of electrons as 18Ar. The ion is Ca2+ (c) Aluminum is a metal in Group 13. It loses three electrons to have the same number of electrons as 10Ne. The ion is Al3+ ...
Solution Preparation Final Goueth
... 33. Which Group III element is expected to have physical and chemical properties that are the least similar to the other elements in that family? (A) B ...
... 33. Which Group III element is expected to have physical and chemical properties that are the least similar to the other elements in that family? (A) B ...
Spin Hall Effect in Cold Atomic Systems
... ¾ Spin Hall effect can be demonstrated in atomic system through optical methods, where the created spin current is conserved and may be able to exhibit topological properties. ¾ Spin Hall effect in cold bosonic atomic gas has very different significance from that in cold fermionic atomic system. In ...
... ¾ Spin Hall effect can be demonstrated in atomic system through optical methods, where the created spin current is conserved and may be able to exhibit topological properties. ¾ Spin Hall effect in cold bosonic atomic gas has very different significance from that in cold fermionic atomic system. In ...
Document
... 2.4 Brønsted Acids and Bases “Brønsted-Lowry” is usually shortened to “Brønsted” • A Brønsted acid is a substance that donates a hydrogen ion (H+) • A Brønsted base is a substance that accepts the H+ ...
... 2.4 Brønsted Acids and Bases “Brønsted-Lowry” is usually shortened to “Brønsted” • A Brønsted acid is a substance that donates a hydrogen ion (H+) • A Brønsted base is a substance that accepts the H+ ...
Chemistry - cloudfront.net
... 19. when forming ions (i.e., cations or anions), know which ions metals usually form and which ions nonmetals usually form 20. understand which parts of the electromagnetic spectrum are high energy/high frequency/short wavelength and which are considered low energy/low frequency/long wavelength 21. ...
... 19. when forming ions (i.e., cations or anions), know which ions metals usually form and which ions nonmetals usually form 20. understand which parts of the electromagnetic spectrum are high energy/high frequency/short wavelength and which are considered low energy/low frequency/long wavelength 21. ...
`universal` phase for electron transmission in quantum dots
... The phase evolution across the first two conductance peaks and valleys (the first two electrons entering the dot) already exhibited a marked deviation from universal behaviour. As demonstrated in the results of two different dot and interferometer designs, the phase climbed by p for each of the firs ...
... The phase evolution across the first two conductance peaks and valleys (the first two electrons entering the dot) already exhibited a marked deviation from universal behaviour. As demonstrated in the results of two different dot and interferometer designs, the phase climbed by p for each of the firs ...
Atomistic description of wave function localization effects in InxGa1
... 2.1 Density functional theory calculations To analyze the impact of isolated In atoms and In atoms sharing the same N atom, we use the density functional theory (DFT) in the framework of the local density approximation (LDA). It is well known that LDA-DFT underestimates the band gap. An improved de ...
... 2.1 Density functional theory calculations To analyze the impact of isolated In atoms and In atoms sharing the same N atom, we use the density functional theory (DFT) in the framework of the local density approximation (LDA). It is well known that LDA-DFT underestimates the band gap. An improved de ...
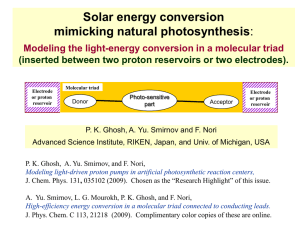
Photosynthesis - Quantum Condensed Matter Research Group
... Kinetic equations can cover from ps to seconds. More importantly, MD solves classical equations, not quantum, and we are studying quantum transport of protons and electrons. ...
... Kinetic equations can cover from ps to seconds. More importantly, MD solves classical equations, not quantum, and we are studying quantum transport of protons and electrons. ...
Principles of Chemistry: A Molecular Approach
... • If a beryllium atom has 4 protons, then it should weigh 4 amu; but it actually weighs 9.01 amu! ...
... • If a beryllium atom has 4 protons, then it should weigh 4 amu; but it actually weighs 9.01 amu! ...
H - Quantum Condensed Matter Research Group
... dynamics ~ ps (up to ~ ms), while kinetic equations (which we use) can cover a far wider range: from ps to seconds. More importantly, MD solves classical equations, not quantum, and we are studying quantum transport of protons and electrons. ...
... dynamics ~ ps (up to ~ ms), while kinetic equations (which we use) can cover a far wider range: from ps to seconds. More importantly, MD solves classical equations, not quantum, and we are studying quantum transport of protons and electrons. ...
Quantum entanglement between the electron clouds of nucleic acids
... The precise value of energy levels is of crucial importance for any kind of interaction in physics. This is also true for processes in biological systems. It has recently been shown for the photosynthesis complex FMO [2–5] that maximum transport efficiency can only be achieved when the environment b ...
... The precise value of energy levels is of crucial importance for any kind of interaction in physics. This is also true for processes in biological systems. It has recently been shown for the photosynthesis complex FMO [2–5] that maximum transport efficiency can only be achieved when the environment b ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.