Lectures 10-11 - U of L Class Index
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
Atomic Structure I. History A. prehistory The four elements B
... Electrons are not arranged haphazardly around the nucleus, but are instead found in specific energy levels he called Quanta = Principle Energy Levels = Periods. ...
... Electrons are not arranged haphazardly around the nucleus, but are instead found in specific energy levels he called Quanta = Principle Energy Levels = Periods. ...
Lectures 10-11
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
Lectures 10-11
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
... For four of the d orbitals, both of these nodes are planes, giving a ‘petal-shaped’ orbital. For the fifth d orbital (_____),the nodes look more like a pair of inverted cones. This gives an orbital that looks a bit like a p orbital with a doughnut around it. (Note the phases, though; they are differ ...
t7_photoel
... Hert’z experiments – speed of radio waves (same as light) 1886-7 Hertz’s observation of the effect of a radio wave on a receiver – photoelectric effect - UV can cause electrons to be emitted from a metal surface (failed to investigate) Experimental results could not be explained by classical wave th ...
... Hert’z experiments – speed of radio waves (same as light) 1886-7 Hertz’s observation of the effect of a radio wave on a receiver – photoelectric effect - UV can cause electrons to be emitted from a metal surface (failed to investigate) Experimental results could not be explained by classical wave th ...
Bonding - Graham ISD
... Atoms combine when the compound formed id more stable than the separate atoms. The only group that seldom forms compounds is the noble gases (group 18). This is true because compounds of these atoms are almost always less stable than the original atom. Atoms with a partially stable outer energy leve ...
... Atoms combine when the compound formed id more stable than the separate atoms. The only group that seldom forms compounds is the noble gases (group 18). This is true because compounds of these atoms are almost always less stable than the original atom. Atoms with a partially stable outer energy leve ...
Units 1-6
... I understand that energy takes different forms, and I can classify energy as potential (chemical, positional, gravitational), kinetic (including temperature) or radiant. I know that temperature is a measure of average kinetic energy I can compare key features of the Fahrenheit, Celsius and Kelvin te ...
... I understand that energy takes different forms, and I can classify energy as potential (chemical, positional, gravitational), kinetic (including temperature) or radiant. I know that temperature is a measure of average kinetic energy I can compare key features of the Fahrenheit, Celsius and Kelvin te ...
Chemistry Test Review - Greenslime Home Page
... b. symbol = K c. atomic mass = 39 d. protons = 19 e. neutrons = 20 f. electrons = 19 g. # of valence electrons = 1 Draw a Bohr model of the element Silicon. a. the element should have: 14 Protons 14 Neutrons ...
... b. symbol = K c. atomic mass = 39 d. protons = 19 e. neutrons = 20 f. electrons = 19 g. # of valence electrons = 1 Draw a Bohr model of the element Silicon. a. the element should have: 14 Protons 14 Neutrons ...
Exam 3 Review
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
HWU4-21 QUESTION: The principal quantum number, n, describes
... The principal quantum number, n, describes the energy level of a particular orbital as a function of the distance from the center of the nucleus. Additional quantum numbers exist to quantify the other characteristics of the electron. The angular momentum quantum number (ℓ), the magnetic quantum numb ...
... The principal quantum number, n, describes the energy level of a particular orbital as a function of the distance from the center of the nucleus. Additional quantum numbers exist to quantify the other characteristics of the electron. The angular momentum quantum number (ℓ), the magnetic quantum numb ...
Many-Electron Atomic States, Terms, and Levels
... a product-like nature. But since we have incorporated the antisymmetric nature of electronic wavefunctions via the Slater Determinantal form, the effect is apparane tin the form of the exchange integrals which arise naturally The total density is not a simple product of each orbital density. The Cou ...
... a product-like nature. But since we have incorporated the antisymmetric nature of electronic wavefunctions via the Slater Determinantal form, the effect is apparane tin the form of the exchange integrals which arise naturally The total density is not a simple product of each orbital density. The Cou ...
The Particulate Nature of Light
... Electrons have been regarded as showing particle-like properties - e.g., the deflection of a stream of electrons is just like the path of a projectile. Electrons can also exhibit wave properties - e.g., they can demonstrate diffraction. De Broglie proposed that an electron of mass m moving with spee ...
... Electrons have been regarded as showing particle-like properties - e.g., the deflection of a stream of electrons is just like the path of a projectile. Electrons can also exhibit wave properties - e.g., they can demonstrate diffraction. De Broglie proposed that an electron of mass m moving with spee ...
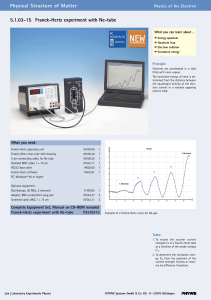
5.1.03-15 Franck-Hertz experiment with Ne
... angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Planck’s constant. Bohr’s picture of electrons in discrete states with transitions among those states producing radiation whose frequency is determined by the energy differences between stat ...
... angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Planck’s constant. Bohr’s picture of electrons in discrete states with transitions among those states producing radiation whose frequency is determined by the energy differences between stat ...
atoms-chemical
... • While all atoms of a given element have the same number of protons (atomic number), they may differ in the number of neutrons and atomic mass. • Two atoms of the same element that differ in the number of neutrons are called isotopes. • For example, 99% of carbon atoms have 6 neutrons (12C). 1% of ...
... • While all atoms of a given element have the same number of protons (atomic number), they may differ in the number of neutrons and atomic mass. • Two atoms of the same element that differ in the number of neutrons are called isotopes. • For example, 99% of carbon atoms have 6 neutrons (12C). 1% of ...
Fall Exam 1
... demonstrated the existence of more than one charge. neutrons. B. proved that Thomson’s “plum D. determined the charge on a single pudding” model of the atom’s electron. structure was correct. 19. Nobel prize winner Ernest Rutherford conducted an experiment with gold foil and alpha particles, leading ...
... demonstrated the existence of more than one charge. neutrons. B. proved that Thomson’s “plum D. determined the charge on a single pudding” model of the atom’s electron. structure was correct. 19. Nobel prize winner Ernest Rutherford conducted an experiment with gold foil and alpha particles, leading ...
Spectra of Atoms
... • The quantum of angular momentum is the same as the proportionality constant in the relation of energy and (angular) frequency: ...
... • The quantum of angular momentum is the same as the proportionality constant in the relation of energy and (angular) frequency: ...
Chemistry Lesson Plans #12
... quantized energies of electrons as they relate to the quantum mechanical model of the atom. - The Evolution of Atomic Models o So far we have described the atom as a nucleus of protons and neutrons surrounded by electrons – this works well for a simple explanation, but does not explain certain prope ...
... quantized energies of electrons as they relate to the quantum mechanical model of the atom. - The Evolution of Atomic Models o So far we have described the atom as a nucleus of protons and neutrons surrounded by electrons – this works well for a simple explanation, but does not explain certain prope ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.