Chemistry Study Guide What is matter made of? Matter is anything
... properties that are the same or very similar. The elements in each group also have the same number of electrons in their outer shell. The horizontal rows are called periods. The elements in each period are arranged by atomic number and have the same number of electron shells around the nucleus. Eac ...
... properties that are the same or very similar. The elements in each group also have the same number of electrons in their outer shell. The horizontal rows are called periods. The elements in each period are arranged by atomic number and have the same number of electron shells around the nucleus. Eac ...
Year 11 Chemistry Balancing Equations
... c When dilute sodium sulfate Na2SO4 solution is added to dilute barium nitrate Ba(NO3)2 solution, barium sulfate BaSO4 precipitates, leaving sodium nitrate NaNO3 in solution. d Dilute sodium hydroxide is added to dilute sulfuric acid H2SO4, producing water and the soluble salt sodium sulfate Na2SO4 ...
... c When dilute sodium sulfate Na2SO4 solution is added to dilute barium nitrate Ba(NO3)2 solution, barium sulfate BaSO4 precipitates, leaving sodium nitrate NaNO3 in solution. d Dilute sodium hydroxide is added to dilute sulfuric acid H2SO4, producing water and the soluble salt sodium sulfate Na2SO4 ...
Modern Atomic Structure
... Energy is quantized. It comes in chunks. A quanta is the amount of energy needed to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. Schrodinger derived an equation that described the energy and position of the ele ...
... Energy is quantized. It comes in chunks. A quanta is the amount of energy needed to move from one energy level to another. Since the energy of an atom is never “in between” there must be a quantum leap in energy. Schrodinger derived an equation that described the energy and position of the ele ...
Atomic and Molecular Spectroscopy
... o Review of basic spectroscopy o Hydrogen energy levels o Fine structure o Spin-orbit coupling o Nuclear moments and hyperfine structure ...
... o Review of basic spectroscopy o Hydrogen energy levels o Fine structure o Spin-orbit coupling o Nuclear moments and hyperfine structure ...
atomicspectra1-2
... • A particular level is denoted either by nlj or by nl 2LJ with L = l and J = j. • The multiplicity of the L term is equal to 2S + 1 = 2s + 1 = 2.: doublet : two levels, with J = L ± 1/2, respectively • The Coulomb interaction between the nucleus and the single electron is dominant, so that the larg ...
... • A particular level is denoted either by nlj or by nl 2LJ with L = l and J = j. • The multiplicity of the L term is equal to 2S + 1 = 2s + 1 = 2.: doublet : two levels, with J = L ± 1/2, respectively • The Coulomb interaction between the nucleus and the single electron is dominant, so that the larg ...
Biochemistry Introduction day 1
... Ex: Oxygen usually has 8 neutrons but 9 and 10 neutrons can be found in some oxygen atoms. Some isotopes are unstable in the nucleus which makes it more likely to decay and release energy. This is RADIO ACTIVITY (radioisotopes) ...
... Ex: Oxygen usually has 8 neutrons but 9 and 10 neutrons can be found in some oxygen atoms. Some isotopes are unstable in the nucleus which makes it more likely to decay and release energy. This is RADIO ACTIVITY (radioisotopes) ...
A commentary on Eric Scerri`s paper “Has Quantum Mechanics
... The correspondence between the spectral properties of atoms and their chemistry was used by Niels Bohr to “deduce” the periodic table (see, e.g., Pais, 1991). In what follows, we’ll concentrate on the electronic properties of atoms as revealed by their spectra. In quantum mechanics, only few problem ...
... The correspondence between the spectral properties of atoms and their chemistry was used by Niels Bohr to “deduce” the periodic table (see, e.g., Pais, 1991). In what follows, we’ll concentrate on the electronic properties of atoms as revealed by their spectra. In quantum mechanics, only few problem ...
Chemistry: The Basics
... mass was 1/1840 H, and the charge was one unit of negative charge. – Actual mass: 9.11 x 10-28 __________ grams ...
... mass was 1/1840 H, and the charge was one unit of negative charge. – Actual mass: 9.11 x 10-28 __________ grams ...
BAND THEORY OF SOLIDS
... isolated, has a discrete set of electron energy levels 1s,2s,2p,....... If we imagine all the N atoms of the solid to be isolated from one another, they would have completely coinciding schemes of their energy levels. Let us study what happens to the energy levels of an isolated atom, as they are br ...
... isolated, has a discrete set of electron energy levels 1s,2s,2p,....... If we imagine all the N atoms of the solid to be isolated from one another, they would have completely coinciding schemes of their energy levels. Let us study what happens to the energy levels of an isolated atom, as they are br ...
pt.1 - MAGNETISM.eu
... From a magnetic viewpoint, the key question is how do the spin and orbital moments of the electrons add together? Magnetism is associated with partly-filled shells, because when the orbitals are all filled with two electrons each with opposite spin there is no spin moment, and when the ± ml orbital ...
... From a magnetic viewpoint, the key question is how do the spin and orbital moments of the electrons add together? Magnetism is associated with partly-filled shells, because when the orbitals are all filled with two electrons each with opposite spin there is no spin moment, and when the ± ml orbital ...
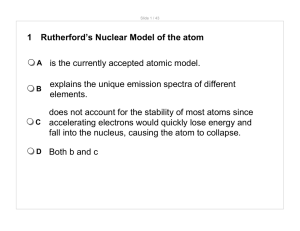
1 Rutherford`s Nuclear Model of the atom A is the currently accepted
... In the quantum-mechanical model of the atom, which of the following is NOT one of the four quantum numbers needed to specify the probable location of an electron? ...
... In the quantum-mechanical model of the atom, which of the following is NOT one of the four quantum numbers needed to specify the probable location of an electron? ...
Answers
... vertical intercept is a measure of the binding energy – called in this case the work function. d) What is the equation of the line and what is the physical meaning of each term? E = hf -W The E refers to the kinetic energy of the fastest emitted electrons. The horizontal variable is the frequency of ...
... vertical intercept is a measure of the binding energy – called in this case the work function. d) What is the equation of the line and what is the physical meaning of each term? E = hf -W The E refers to the kinetic energy of the fastest emitted electrons. The horizontal variable is the frequency of ...
Energy Levels and Light Absorption
... • Consider an electron in an atom with quantum number n = n1. How many electrons in this atom can have the quantum number n1? n1 • Pauli exclusion says no two electrons can be in exactly the same state ...
... • Consider an electron in an atom with quantum number n = n1. How many electrons in this atom can have the quantum number n1? n1 • Pauli exclusion says no two electrons can be in exactly the same state ...
Environmental Physics for Freshman Geography Students
... Planck’s constant is the underlying numerical constant behind so-called quantum mechanics; that is the mechanics that describes the way molecules, atoms and other small-scale systems interact with one another. One of the unfortunate aspects of modern physics is that there is no known simple way to p ...
... Planck’s constant is the underlying numerical constant behind so-called quantum mechanics; that is the mechanics that describes the way molecules, atoms and other small-scale systems interact with one another. One of the unfortunate aspects of modern physics is that there is no known simple way to p ...
Lect 23 Presentation
... To be consistent with the Heisenberg Uncertainty Principle, which of these properties can not be quantized (have the exact value known)? (more than one answer can be correct) Electron Orbital Radius ...
... To be consistent with the Heisenberg Uncertainty Principle, which of these properties can not be quantized (have the exact value known)? (more than one answer can be correct) Electron Orbital Radius ...
The Development of a New Atomic Model
... Rutherford- did not explain how the atom’s negatively charged particles are distributed in the space surrounding it’s positively charged nucleus. ...
... Rutherford- did not explain how the atom’s negatively charged particles are distributed in the space surrounding it’s positively charged nucleus. ...
Pre-AP Chemistry
... 12. scientific notation 13. use of proportions 14. particle and atomic theory 15. atomic laws- Conservation of Mass, Definite Proportions, Multiple Proportions 16. subatomic atomic particles 17. regions of the atom 18. atomic symbols, numbers, masses 19. average atomic masses by abundance 20. Avogad ...
... 12. scientific notation 13. use of proportions 14. particle and atomic theory 15. atomic laws- Conservation of Mass, Definite Proportions, Multiple Proportions 16. subatomic atomic particles 17. regions of the atom 18. atomic symbols, numbers, masses 19. average atomic masses by abundance 20. Avogad ...
Frank-Hertz experiment with Neon
... The Franck-Hertz experiment was a physics experiment that provided support for the Bohr model of the atom, a precursor to quantum mechanics. In 1914, the German physicists James Franck and Gustav Ludwig Hertz sought to experimentally probe the energy levels of the atom. The now-famous Franck-Hertz e ...
... The Franck-Hertz experiment was a physics experiment that provided support for the Bohr model of the atom, a precursor to quantum mechanics. In 1914, the German physicists James Franck and Gustav Ludwig Hertz sought to experimentally probe the energy levels of the atom. The now-famous Franck-Hertz e ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.