So where did all the matter on Earth come from - Bennatti
... atomic number of helium is two. Each helium atom has two protons. No other element is made of atoms with two protons in the nucleus. Each element is represented with a chemical symbol. Most chemical symbols are one or two letters. The first letter is always capitalized. If it has two or three letter ...
... atomic number of helium is two. Each helium atom has two protons. No other element is made of atoms with two protons in the nucleus. Each element is represented with a chemical symbol. Most chemical symbols are one or two letters. The first letter is always capitalized. If it has two or three letter ...
Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum
... We can now extend the Rigid Rotor problem to a rotation in 3D, corresponding to motion on the surface of a sphere of radius R. The Hamiltonian operator in this case is derived from the Laplacian in spherical polar coordinates given as ...
... We can now extend the Rigid Rotor problem to a rotation in 3D, corresponding to motion on the surface of a sphere of radius R. The Hamiltonian operator in this case is derived from the Laplacian in spherical polar coordinates given as ...
Matter
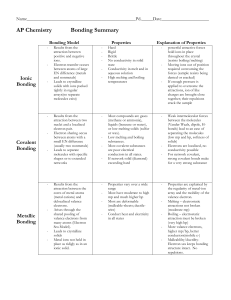
... properties from the elements of which they are composed. Chemical bonds are the forces that hold the elements together in a compound creating a state of stability. ...
... properties from the elements of which they are composed. Chemical bonds are the forces that hold the elements together in a compound creating a state of stability. ...
T1_The_Origins_Of_Quantum_Mechanics
... observed features are also caused by absorption in oxygen molecules in the Earth's atmosphere. ...
... observed features are also caused by absorption in oxygen molecules in the Earth's atmosphere. ...
Chemistry Test Study Guide
... 21. A mixture is created when two pure substances are combined so that each of the pure substances retains its own properties. 22. Where is the majority of the mass of an atom located? In the nucleus.(Protons and Neutrons) 23. If an atom loses electron’s, will it have a positive or negative charge? ...
... 21. A mixture is created when two pure substances are combined so that each of the pure substances retains its own properties. 22. Where is the majority of the mass of an atom located? In the nucleus.(Protons and Neutrons) 23. If an atom loses electron’s, will it have a positive or negative charge? ...
Quantum Physics Part II Quantum Physics in three units Bright Line
... • A new quantum number was used. It was called the l quantum number or the azimuthal quantum number. • These were called subshells. • For any given quantum number n, the possible subshells range from l=0 to l=n-1 • Again, the angular momentum was determined by the value according to ...
... • A new quantum number was used. It was called the l quantum number or the azimuthal quantum number. • These were called subshells. • For any given quantum number n, the possible subshells range from l=0 to l=n-1 • Again, the angular momentum was determined by the value according to ...
+1/2 and
... geometric place of points with a given r value build a sphere. An orbital has n-l spheric nodal surfaces, one of them is in the infinity. The points with common q build a cone; at 90o cosq=0, cone deforms to a plane; their number is: l - m The points with common polar angle build planes (sin=0 or c ...
... geometric place of points with a given r value build a sphere. An orbital has n-l spheric nodal surfaces, one of them is in the infinity. The points with common q build a cone; at 90o cosq=0, cone deforms to a plane; their number is: l - m The points with common polar angle build planes (sin=0 or c ...
Chemistry Semester One Exam Review Name:
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
Study On the Capacitance Between Orbitals and Atoms Modeling
... Since 100 year, Niels Bohr suggested his model of the atom. He succeeded only in interpretation of line spectrum of single electron atoms (Hydrogen and ionized Helium) so more general model was still needed. In 1926, Erwin Schrödinger presented his famous equation which described complicated physica ...
... Since 100 year, Niels Bohr suggested his model of the atom. He succeeded only in interpretation of line spectrum of single electron atoms (Hydrogen and ionized Helium) so more general model was still needed. In 1926, Erwin Schrödinger presented his famous equation which described complicated physica ...
Quantum Numbers Activity
... Quantum Numbers • Used to describe various properties of the orbitals • Each electron is assigned a set of four quantum numbers which, in order, are n, l, ml , and ms • Like giving each electron its own address ...
... Quantum Numbers • Used to describe various properties of the orbitals • Each electron is assigned a set of four quantum numbers which, in order, are n, l, ml , and ms • Like giving each electron its own address ...
Learning Goals - Issaquah Connect
... Go to the list of Phet HTML5 Chemistry simulations. Click on the Build an Atom simulation and start the sim. Once the simulation opens, click on “Atom”. a. Click on the X’s behind the Net Charge and Mass Number titles to display the graphics. Add protons, neutrons & electrons to the model until you ...
... Go to the list of Phet HTML5 Chemistry simulations. Click on the Build an Atom simulation and start the sim. Once the simulation opens, click on “Atom”. a. Click on the X’s behind the Net Charge and Mass Number titles to display the graphics. Add protons, neutrons & electrons to the model until you ...
wlq10
... • Messenger series of lectures, Cornell University, 1964 • Lecture 6: ‘Probability and Uncertainty – the quantum mechanical view of nature’ • The Character of Physical Law - Penguin • see the later series of Douglas Robb memorial lectures (1979) online ...
... • Messenger series of lectures, Cornell University, 1964 • Lecture 6: ‘Probability and Uncertainty – the quantum mechanical view of nature’ • The Character of Physical Law - Penguin • see the later series of Douglas Robb memorial lectures (1979) online ...
... When electrons are confined to a small region of a semiconductor they form a quantum dot, and the energy and the charge on the quantum dot are quantized. It has been possible to study the transmission of electrons through a quantum dot by coupling the states in the dot to external leads via a tunnel ...
lecture notes, pages 4-5
... Microscopic particles, like electrons, whose �’s are on the order of their environment do not obey classical equations of motion. Electrons must be treated like waves to describe their behavior. 1927 Erwin Schrödinger wrote an equation of motion for particles (like electrons) that account for their ...
... Microscopic particles, like electrons, whose �’s are on the order of their environment do not obey classical equations of motion. Electrons must be treated like waves to describe their behavior. 1927 Erwin Schrödinger wrote an equation of motion for particles (like electrons) that account for their ...
Chp 1,2 rev
... How many grams are in 100ml of a solution with a density of 2.5g/ml? Describe Solids, Liquids, and Gases. Calculate the volume of 15 g of a solid with density of 6g/ml. ...
... How many grams are in 100ml of a solution with a density of 2.5g/ml? Describe Solids, Liquids, and Gases. Calculate the volume of 15 g of a solid with density of 6g/ml. ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.