Lab Handout
... Show all set-ups for each type of calculation: be explicit. If you have to perform the same calculation more than once you do not have to write the set-up for each one, but it should be clear as to which set-up correlates to which calculation. An example follows. The solubility experiment discussed ...
... Show all set-ups for each type of calculation: be explicit. If you have to perform the same calculation more than once you do not have to write the set-up for each one, but it should be clear as to which set-up correlates to which calculation. An example follows. The solubility experiment discussed ...
RESEARCH ARTICLE Bottles as models
... Gill size was increased to 1.5cm width, representing the enlarged gill slits of the basking shark, buccal length was decreased (to 15cm total, 12cm buccal) to test for differences in ontogeny, and flow speed (fish swimming speed) was increased to 60cms–1 for each gill number variation. Adjustin ...
... Gill size was increased to 1.5cm width, representing the enlarged gill slits of the basking shark, buccal length was decreased (to 15cm total, 12cm buccal) to test for differences in ontogeny, and flow speed (fish swimming speed) was increased to 60cms–1 for each gill number variation. Adjustin ...
Electrostatic lattice with alternating
... RF on: p / p p / p max cos z Idea of using the RF cavity was expressed long time ago by many authors, for instance [ A.P. Lysenko et al., Part.Accel. 18, 215 (1986) ]. ...
... RF on: p / p p / p max cos z Idea of using the RF cavity was expressed long time ago by many authors, for instance [ A.P. Lysenko et al., Part.Accel. 18, 215 (1986) ]. ...
Calculation of the nucleon axial charge in lattice QCD
... Quantum Chromodynamics is the theory of the Strong Force of nature. The theory dictates how quarks, the fundamental components of ordinary matter, interact by exchanging particles known as gluons. The interactions between quarks and gluons are sufficiently strong to bind them into bound states such ...
... Quantum Chromodynamics is the theory of the Strong Force of nature. The theory dictates how quarks, the fundamental components of ordinary matter, interact by exchanging particles known as gluons. The interactions between quarks and gluons are sufficiently strong to bind them into bound states such ...
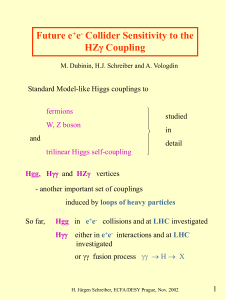
e + e
... • no further particle in a cone of half-angle 10o • if more than 1 photon, the one with largest energy is selected • all remaining particles 2 jets • Mjj MZ (84 - 105 GeV) ...
... • no further particle in a cone of half-angle 10o • if more than 1 photon, the one with largest energy is selected • all remaining particles 2 jets • Mjj MZ (84 - 105 GeV) ...
chapter 2 photons and atoms
... Photon Time A photon in a monochromatic mode is equally likely to be detected at any time. A general expansion may be made in terms of polychromatic modes (timelocalized wavepackets). The probability of detecting the photon described by the complex wavefunction U(r, t), at any position, in the incre ...
... Photon Time A photon in a monochromatic mode is equally likely to be detected at any time. A general expansion may be made in terms of polychromatic modes (timelocalized wavepackets). The probability of detecting the photon described by the complex wavefunction U(r, t), at any position, in the incre ...
Path Project Presentation - atlas-com-media.web.cern.ch
... concept of the exhibition is to invite visitors to participate in a game, allowing them to cross the detector as particles, interacting with parts of it. Visitors are first presented with a selection of the Big Questions facing mankind in our understanding of the physical universe. They are then ...
... concept of the exhibition is to invite visitors to participate in a game, allowing them to cross the detector as particles, interacting with parts of it. Visitors are first presented with a selection of the Big Questions facing mankind in our understanding of the physical universe. They are then ...
Clive_Speake
... • Ideas beyond the Standard Model of Particle physics and, perhaps, also that of Cosmology are needed to make sense of gravity. • Searches for new weak interactions are complementary to direct searches for new bosons in particle accelerators. • Fundamental physics experiments in the lab or, perhaps, ...
... • Ideas beyond the Standard Model of Particle physics and, perhaps, also that of Cosmology are needed to make sense of gravity. • Searches for new weak interactions are complementary to direct searches for new bosons in particle accelerators. • Fundamental physics experiments in the lab or, perhaps, ...
the periodic table of elementary particles
... have integer electric charges and hypercharges, while quarks have fractional electric charges and hypercharges. The leptonization is to make quarks behave like leptons in terms of "apparent" integer electric charges and hypercharges. Obviously, the result of such a leptonization is to create the str ...
... have integer electric charges and hypercharges, while quarks have fractional electric charges and hypercharges. The leptonization is to make quarks behave like leptons in terms of "apparent" integer electric charges and hypercharges. Obviously, the result of such a leptonization is to create the str ...
NUFACT11-Blondel-goals-of-the-workshop
... 1. Is Project X a suitable proton driver for the Neutrino Factory? 2. What is the path for solving the problem of operating high gradient RF is strong magnetic field? ...
... 1. Is Project X a suitable proton driver for the Neutrino Factory? 2. What is the path for solving the problem of operating high gradient RF is strong magnetic field? ...
Stability of Few-Charge Systems in Quantum Mechanics
... In classical mechanics, determining the motion of three bodies which attract each other according to Newton’s universal law of gravitation is the most celebrated of all dynamical problems (see, for example, [1], [2]). Over the period since 1750, it has attracted the attention of some of the greatest ...
... In classical mechanics, determining the motion of three bodies which attract each other according to Newton’s universal law of gravitation is the most celebrated of all dynamical problems (see, for example, [1], [2]). Over the period since 1750, it has attracted the attention of some of the greatest ...
Day 1(1/8)-
... units of work [W]=J(joule)=Nm(newton-meter) Hwk - p.227 #1-4 + 5pt assignment to email [email protected] Day 2(1/10)-Extending our concept of work... The force must be in the direction of the displacement. 0J of work is possible. Friction usually does 'negative work'. The force of gravity does pos ...
... units of work [W]=J(joule)=Nm(newton-meter) Hwk - p.227 #1-4 + 5pt assignment to email [email protected] Day 2(1/10)-Extending our concept of work... The force must be in the direction of the displacement. 0J of work is possible. Friction usually does 'negative work'. The force of gravity does pos ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.