1 This is the pre-review version of the paper published in Journal of
... In the following discussion, vibrational effects have been neglected in order to focus on the electronic effects: unlike the relaxed molecules, the strained conformations of the analyzed vinylogues are not minima in their potential energy landscapes, and therefore inclusion of vibrational effects wo ...
... In the following discussion, vibrational effects have been neglected in order to focus on the electronic effects: unlike the relaxed molecules, the strained conformations of the analyzed vinylogues are not minima in their potential energy landscapes, and therefore inclusion of vibrational effects wo ...
The breakdown of alcohol and your body How do you prevent a
... (artificial) colors, flavors, sulfites and other additives that are added to certain alcoholic beverages. Not drinking enough water has a greater influence on the severity of a hangover. Alcohol makes your kidneys excrete more water than usual, making you urinate more often. If not compensated by dr ...
... (artificial) colors, flavors, sulfites and other additives that are added to certain alcoholic beverages. Not drinking enough water has a greater influence on the severity of a hangover. Alcohol makes your kidneys excrete more water than usual, making you urinate more often. If not compensated by dr ...
Word Version of Answer Key
... 2. After the ADH converts ethanol to acetaldehyde, another enzyme called acetaldehyde dehydrogenase (ALDH) further breaks down the acetaldehyde into acetate as shown here: C2H4O→C2H3O- ...
... 2. After the ADH converts ethanol to acetaldehyde, another enzyme called acetaldehyde dehydrogenase (ALDH) further breaks down the acetaldehyde into acetate as shown here: C2H4O→C2H3O- ...
How is Alcohol Metabolized?
... 2. After the ADH converts ethanol to acetaldehyde, another enzyme called acetaldehyde dehydrogenase (ALDH) further breaks down the acetaldehyde into acetate as shown here: C2H4O→C2H3O- ...
... 2. After the ADH converts ethanol to acetaldehyde, another enzyme called acetaldehyde dehydrogenase (ALDH) further breaks down the acetaldehyde into acetate as shown here: C2H4O→C2H3O- ...
Word Version of Answer Key
... increased that production rather than the production of ALDH and over time this need has been genetically passed down. There will be a variety of answers for this question. 5. The next step in the breakdown of alcohol is for the body to break down acetic acid into carbon dioxide (CO2) and water (H2O ...
... increased that production rather than the production of ALDH and over time this need has been genetically passed down. There will be a variety of answers for this question. 5. The next step in the breakdown of alcohol is for the body to break down acetic acid into carbon dioxide (CO2) and water (H2O ...
QUESTIONS FOR PRACTICE HYDROCARBONS 1. Name the least
... 18. Explain with an example the reaction of aldehydes with sodium bisulphite (NaHSO3). 19. Explain with an example the reaction of ketones with sodium bisulphite (NaHSO3). 20. How do convert acetaldehyde to crotonaldehyde? 21. How does acetaldehyde react with Tollen`s reagent? 22. How does acetaldeh ...
... 18. Explain with an example the reaction of aldehydes with sodium bisulphite (NaHSO3). 19. Explain with an example the reaction of ketones with sodium bisulphite (NaHSO3). 20. How do convert acetaldehyde to crotonaldehyde? 21. How does acetaldehyde react with Tollen`s reagent? 22. How does acetaldeh ...
PDF Version
... 4. Some ethnic groups have very few ALDH enzymes, which causes them to have adverse effects of alcohol consumption, such as flushed skin soon after drinking. What biological phenomena could explain why certain ethnic groups have so few of these enzymes? (Think about things like genetics and evolutio ...
... 4. Some ethnic groups have very few ALDH enzymes, which causes them to have adverse effects of alcohol consumption, such as flushed skin soon after drinking. What biological phenomena could explain why certain ethnic groups have so few of these enzymes? (Think about things like genetics and evolutio ...
Living with Alcohol by Steven Wm. Fowkes
... the stomach into its component amino acids (glutamate, cysteine and glycine). So while glutathione is a great idea, it’s an expensive great idea. Thiamine (vitamin B-1) and lipoic (thioctic) acid are key sulfur-containing nutrients that may be depleted by alcohol and/or may help with acetaldehyde d ...
... the stomach into its component amino acids (glutamate, cysteine and glycine). So while glutathione is a great idea, it’s an expensive great idea. Thiamine (vitamin B-1) and lipoic (thioctic) acid are key sulfur-containing nutrients that may be depleted by alcohol and/or may help with acetaldehyde d ...
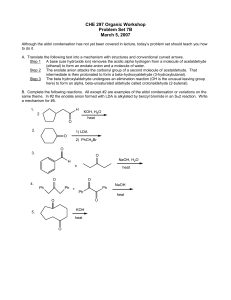
CHE 297 Organic Workshop
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
... (ethanal) to form an enolate anion and a molecule of water. Step 2 The enolate anion attacks the carbonyl group of a second molecule of acetaldehyde. That intermediate is then protonated to form a beta-hydroxyaldehyde (3-hydroxybutanal). Step 3 The beta hydroxylaldehyde undergoes an elimination reac ...
Acetaldehyde

Acetaldehyde (systematic name ethanal) is an organic chemical compound with the formula CH3CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and may be a contributing factor to hangovers from alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen.