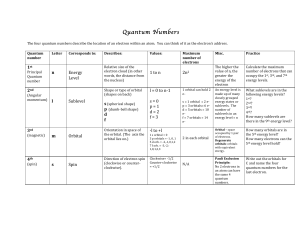
Atom is a basic unit of matter that consists of a nucleus
... species. In 1913, physicist Niels Bohr suggested that the electrons were confined into clearly defined, quantized orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states. An electron must absorb or emit specific amounts of energy to transition betwe ...
... species. In 1913, physicist Niels Bohr suggested that the electrons were confined into clearly defined, quantized orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states. An electron must absorb or emit specific amounts of energy to transition betwe ...
CS378 - M375T - PHY341 Introduction to Quantum
... Canvas page at http://canvas.utexas.edu/. If you prefer to write your solutions by hand, you can upload hi-res photos of your solutions (e.g., using a mobile phone). Otherwise, you can type solutions in Word, LaTeX, or other software of your choice. A single problem set (the one with the lowest scor ...
... Canvas page at http://canvas.utexas.edu/. If you prefer to write your solutions by hand, you can upload hi-res photos of your solutions (e.g., using a mobile phone). Otherwise, you can type solutions in Word, LaTeX, or other software of your choice. A single problem set (the one with the lowest scor ...
Fall 2010
... Quantum mechanics provides a mathematical description of the behavior and interactions of very small particles that are not correctly described by classical mechanics. This course is designed to provide students with the knowledge, theoretical background and mathematical tools to understand theoreti ...
... Quantum mechanics provides a mathematical description of the behavior and interactions of very small particles that are not correctly described by classical mechanics. This course is designed to provide students with the knowledge, theoretical background and mathematical tools to understand theoreti ...
Principles of Computer Architecture Dr. Mike Frank
... – Because two such swaps gives the identical quantum state, UU=1 (identity operation), – One swap U must multiply the state vector by 1. – There are only two square roots of 1: Namely, 1 and 1. ...
... – Because two such swaps gives the identical quantum state, UU=1 (identity operation), – One swap U must multiply the state vector by 1. – There are only two square roots of 1: Namely, 1 and 1. ...
Electron spin and the periodic table
... (Actually, the original experiment used silver atoms, but their moment comes from the spin of an unpaired electron, so it is basically the same thing.) In the experiments, it is found that the actual moment is μz = - e ħ / 2 m, so it is just twice the expected value. This was called the anomalous mo ...
... (Actually, the original experiment used silver atoms, but their moment comes from the spin of an unpaired electron, so it is basically the same thing.) In the experiments, it is found that the actual moment is μz = - e ħ / 2 m, so it is just twice the expected value. This was called the anomalous mo ...
polarization of the allotropic hollow foms of carbon and its use in
... Technological Institute of Superhard and Novel Carbon Materials, Moscow, Russian Federation [email protected] The analytical model of polarizing resonant interactions hollow forms of carbon with the quantum charged particles with total energy E > 0 is offered. The problem is shown to classical quant ...
... Technological Institute of Superhard and Novel Carbon Materials, Moscow, Russian Federation [email protected] The analytical model of polarizing resonant interactions hollow forms of carbon with the quantum charged particles with total energy E > 0 is offered. The problem is shown to classical quant ...
Slide 1
... Frequency, : The number of wave peaks that pass a given point per unit time (1/s) Wavelength, : The distance from one wave peak to the next (nm or m) Amplitude: Height of wave ...
... Frequency, : The number of wave peaks that pass a given point per unit time (1/s) Wavelength, : The distance from one wave peak to the next (nm or m) Amplitude: Height of wave ...
Creation and Annihilation Operators
... because (11) tells us that (fj† )2 = 0. Thus there are no states with two fermions in the same orbital. • The state produced by applying fj† fk† to the vacuum differs from that obtained using fk† fj† by a minus sign. Thus the two are not linearly independent, and only one should enter in a list of b ...
... because (11) tells us that (fj† )2 = 0. Thus there are no states with two fermions in the same orbital. • The state produced by applying fj† fk† to the vacuum differs from that obtained using fk† fj† by a minus sign. Thus the two are not linearly independent, and only one should enter in a list of b ...
Quantum Mechanics
... 2. The hydrogen atom wave function may be written as R(r)Y`m (θ, φ), where R is the radial function and Y`m are the spherical harmonics. a. What is the differential equation for R(r)? b. The differential equation may be simplified somewhat by changing r and E into ρ = r/a0 and W = E/[ke2 /(2a0 )], w ...
... 2. The hydrogen atom wave function may be written as R(r)Y`m (θ, φ), where R is the radial function and Y`m are the spherical harmonics. a. What is the differential equation for R(r)? b. The differential equation may be simplified somewhat by changing r and E into ρ = r/a0 and W = E/[ke2 /(2a0 )], w ...
Section 7.1
... case of square systems, in which n = m, as these occur most frequently in practice. ...
... case of square systems, in which n = m, as these occur most frequently in practice. ...
Variational principle in the conservation operators deduction
... where is the certain constant which value dependent on our choice of this or that unit system only. One can notice that has the velocity dimension. It is easy to show (see the Appendix) that means the velocity of propagation of the studied object in the case of inertial movement. Thus we have ...
... where is the certain constant which value dependent on our choice of this or that unit system only. One can notice that has the velocity dimension. It is easy to show (see the Appendix) that means the velocity of propagation of the studied object in the case of inertial movement. Thus we have ...

















![[30 pts] While the spins of the two electrons in a hydrog](http://s1.studyres.com/store/data/002487557_1-ac2bceae20801496c3356a8afebed991-300x300.png)