Resonant Tunneling Between Quantum Hall Edge States
... Many body correlations often play an important role in tunneling and resonant tunneling in mesoscopic structures, such as quantum dots [1,2]. In addition to the Coulomb correlations in the vicinity of a tunneling structure, it has recently been emphasized that the electron interactions in the “lead ...
... Many body correlations often play an important role in tunneling and resonant tunneling in mesoscopic structures, such as quantum dots [1,2]. In addition to the Coulomb correlations in the vicinity of a tunneling structure, it has recently been emphasized that the electron interactions in the “lead ...
11 Two and many electron atoms - FU Berlin
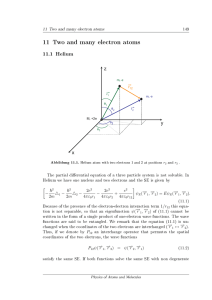
... than the singlet states, due to the reduced electron-electron repulsion. The triplet state (1s)(2s) is metastable, since relaxation to (1s)2 is not possible upon light emission (intercombination lines are forbidden). Excitation of triplet states is possible upon collisions of atoms, spin-orbit coupl ...
... than the singlet states, due to the reduced electron-electron repulsion. The triplet state (1s)(2s) is metastable, since relaxation to (1s)2 is not possible upon light emission (intercombination lines are forbidden). Excitation of triplet states is possible upon collisions of atoms, spin-orbit coupl ...
Manipulation of electron spin in a quantum dot D. G
... the coupling between the dot and the contacts [6]. Quantum algorithms usually assume that either the system dynamically evolves through a sequence of unitary transformations or that a set λ of external control parameters of the Hamiltonian H smoothly changes in time (“adiabatic evolution”) [7]. Acci ...
... the coupling between the dot and the contacts [6]. Quantum algorithms usually assume that either the system dynamically evolves through a sequence of unitary transformations or that a set λ of external control parameters of the Hamiltonian H smoothly changes in time (“adiabatic evolution”) [7]. Acci ...
Lecture notes, Chapter 4. Energy Levels
... The simplest system to be analyzed is a particle in a box: classically, in 3D, the particle is stuck inside the box and can never leave. Another classical analogy would be a ball at the bottom of a well so deep that no matter how much kinetic energy the ball possess, it will never be able to exit th ...
... The simplest system to be analyzed is a particle in a box: classically, in 3D, the particle is stuck inside the box and can never leave. Another classical analogy would be a ball at the bottom of a well so deep that no matter how much kinetic energy the ball possess, it will never be able to exit th ...
Tunneling between Edge States in a Quantum Spin Hall System
... draws on the exploration of topologically nontrivial quantum states. Experimentally realized examples, which are by now well understood, are given by the integer [1] and fractional [2] quantum Hall states. These states defy a classification in terms of the standard Ginzburg-Landau theory of symmetry ...
... draws on the exploration of topologically nontrivial quantum states. Experimentally realized examples, which are by now well understood, are given by the integer [1] and fractional [2] quantum Hall states. These states defy a classification in terms of the standard Ginzburg-Landau theory of symmetry ...
talk
... Einstein et al. assume a limited interaction range of the 2 systems. Outside this range the 2 atoms have nothing to do with each other. We apply the EPR view in a very naive way (Einstein was not that naive!). Immediately after the disintegration of the H2 molecule the two H-atoms are still in the e ...
... Einstein et al. assume a limited interaction range of the 2 systems. Outside this range the 2 atoms have nothing to do with each other. We apply the EPR view in a very naive way (Einstein was not that naive!). Immediately after the disintegration of the H2 molecule the two H-atoms are still in the e ...
Coupled quantum dots as quantum gates
... terdot distance, i.e., for 2a@2a B , where a is half the distance between the centers of the dots, and a B5 A\/m v 0 is the effective Bohr radius of a single isolated harmonic well. This choice for the potential is motivated by the experimental fact5 that the spectrum of single dots in GaAs is well ...
... terdot distance, i.e., for 2a@2a B , where a is half the distance between the centers of the dots, and a B5 A\/m v 0 is the effective Bohr radius of a single isolated harmonic well. This choice for the potential is motivated by the experimental fact5 that the spectrum of single dots in GaAs is well ...
test 3 practice
... ____ 36. What energy must be added or given off in a reaction where two hydrogen atoms and two neutrons are combined to form a helium atom? (Atomic masses for each: hydrogen, 1.007 825 u; neutron, 1.008 665 u; helium, 4.002 602 u; also, 1 u = 931.5 MeV/c2) a. 20.7 MeV added b. 20.7 MeV given off c. ...
... ____ 36. What energy must be added or given off in a reaction where two hydrogen atoms and two neutrons are combined to form a helium atom? (Atomic masses for each: hydrogen, 1.007 825 u; neutron, 1.008 665 u; helium, 4.002 602 u; also, 1 u = 931.5 MeV/c2) a. 20.7 MeV added b. 20.7 MeV given off c. ...
File
... c) Proton: a sub-atomic particle that is found it nucleus of an atom. Protons have a charge of 1+ and a mass of 1 amu. d) Neutron: a sub-atomic particle that is found in the nucleus of an atom. Neutrons have no charge and have a mass of 1 amu. e) Electron: a sub-atomic particle that is found in the ...
... c) Proton: a sub-atomic particle that is found it nucleus of an atom. Protons have a charge of 1+ and a mass of 1 amu. d) Neutron: a sub-atomic particle that is found in the nucleus of an atom. Neutrons have no charge and have a mass of 1 amu. e) Electron: a sub-atomic particle that is found in the ...
Advances in Atomic Physics: An Overview (793 Pages) - Beck-Shop
... approach followed in part 2 provides new physical insights into radiative corrections produced by comparing the perturbations due to a non-zero applied field to those obtained when the electromagnetic field is in its vacuum state. Part 3 - Multiphoton processes An atom can make a transition between ...
... approach followed in part 2 provides new physical insights into radiative corrections produced by comparing the perturbations due to a non-zero applied field to those obtained when the electromagnetic field is in its vacuum state. Part 3 - Multiphoton processes An atom can make a transition between ...
Final
... Please circle all final answers. For full credit you must report all responses to the correct number of significant figures. Include units and substances where appropriate. You may use only the basic arithmetic functions of ...
... Please circle all final answers. For full credit you must report all responses to the correct number of significant figures. Include units and substances where appropriate. You may use only the basic arithmetic functions of ...
A New Form of Matter (pdf, 217 kB)
... Technology) and Carl Wieman (University of Colorado) also created BECs; theirs were made of super-cold rubidium atoms. Cornell and Wieman shared the 2001 Nobel Prize with Ketterle "for the achievement of Bose-Einstein condensation in dilute gases of alkali atoms, and for early fundamental studies of ...
... Technology) and Carl Wieman (University of Colorado) also created BECs; theirs were made of super-cold rubidium atoms. Cornell and Wieman shared the 2001 Nobel Prize with Ketterle "for the achievement of Bose-Einstein condensation in dilute gases of alkali atoms, and for early fundamental studies of ...
Shell Structure of Nuclei and Cold Atomic Gases in Traps
... Cold Fermionic Atoms in 2D Traps – Pairing versus Hund’s Rule ...
... Cold Fermionic Atoms in 2D Traps – Pairing versus Hund’s Rule ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.