if on the Internet, Press on your browser to
... The stationary states of a chaotic system have surprisingly interesting structure as demonstrated in the early 1980s by Eric Heller of the University of Washington. He and his students calculated a series of stationary states for a 2-dimensional cavity in the shape of a stadium. The corresponding p ...
... The stationary states of a chaotic system have surprisingly interesting structure as demonstrated in the early 1980s by Eric Heller of the University of Washington. He and his students calculated a series of stationary states for a 2-dimensional cavity in the shape of a stadium. The corresponding p ...
Semiclassical methods in solid state physics : two examples
... Journal de Physique I, EDP Sciences, 1993, 3 (2), pp.471-499. <10.1051/jp1:1993145>.
...
... Journal de Physique I, EDP Sciences, 1993, 3 (2), pp.471-499. <10.1051/jp1:1993145>.
Easy understanding on Hanle effect No.1 atomic polarization and
... • Atomic (quantum) coherence is non-diagonal elements of
atomic density matrix ρ = ∑ |m> Pm , not the amplitude of |m>!
• If we have complete quantum mechanical description on the whole
system namely atoms and radiation field |radiation field, a ...
... • Atomic (quantum) coherence is non-diagonal elements
Chapter 4
... • Each element is represented by its symbol. • The number of each type of atom is indicated by a subscript written to the right of the element symbol. • If only one atom is present, do not include a subscript. • If polyatomic groups are present in the molecule, they are written inside parentheses if ...
... • Each element is represented by its symbol. • The number of each type of atom is indicated by a subscript written to the right of the element symbol. • If only one atom is present, do not include a subscript. • If polyatomic groups are present in the molecule, they are written inside parentheses if ...
Radio Serial : From Atoms To Stars Episode 11 : Chemical Reactions And the Atom ….
... Raanaa : Right, the balanced chemical equation !! Here, the word balanced is important. Is not it ?? Maainaa : Ya, because combination of different atoms is governed by some fundamental laws of chemical combination …. Raanaa : ….. such as …… ?? Maaina ...
... Raanaa : Right, the balanced chemical equation !! Here, the word balanced is important. Is not it ?? Maainaa : Ya, because combination of different atoms is governed by some fundamental laws of chemical combination …. Raanaa : ….. such as …… ?? Maaina ...
Including Nuclear Degrees of Freedom in a Lattice Hamiltonian, P. L. Hagelstein, I. U. Chaudhary, This paper has been accepted for publication in J. Cond. Mat. Nucl. Sci. and will be published soon. An earlier version was posted on the LANL ArXiV (/0401667 [cond-mat.other] 20 Jan 2012).
... there were no physical transitions which could serve as the strongly coupled two-level transition within the model. We were optimistic in our writing about the possibility that systems described by three-level systems (or N-level systems) would be able to do the job. After putting in a great deal of ...
... there were no physical transitions which could serve as the strongly coupled two-level transition within the model. We were optimistic in our writing about the possibility that systems described by three-level systems (or N-level systems) would be able to do the job. After putting in a great deal of ...
Proposal for Translational Entanglement of Dipole
... solution: (a) controlling the diatom formation and dissociation by switching on and off the LIDDI; (b) controlling the motion and effective masses of the atoms and the diatom by changing the intensities of the lattice fields. System specification.—Let us assume two overlapping optical lattices with ...
... solution: (a) controlling the diatom formation and dissociation by switching on and off the LIDDI; (b) controlling the motion and effective masses of the atoms and the diatom by changing the intensities of the lattice fields. System specification.—Let us assume two overlapping optical lattices with ...
Spin and uncertainty in the interpretation of quantum mechanics
... It can be seen from Eq. (A30) that h r × mu i is the expected angular momentum as defined by the usual “orbital angular momentum operators” in the Schrödinger and Pauli theories. We shall deemphasize the conventional “operator language” in quantum mechanics, because it obscures the relation of exp ...
... It can be seen from Eq. (A30) that h r × mu i is the expected angular momentum as defined by the usual “orbital angular momentum operators” in the Schrödinger and Pauli theories. We shall deemphasize the conventional “operator language” in quantum mechanics, because it obscures the relation of exp ...
Theory of shot noise in high-current space-charge-limited
... where the Dvac共p , x兲 term is the longitudinal self-consistent field describing the screened Coulomb interaction between two charges at position x and x⬘, and p is the wave vector along the x direction. The formulation is basically by solving the screened Poisson equation in its Green’s function for ...
... where the Dvac共p , x兲 term is the longitudinal self-consistent field describing the screened Coulomb interaction between two charges at position x and x⬘, and p is the wave vector along the x direction. The formulation is basically by solving the screened Poisson equation in its Green’s function for ...
Coulomb blockade in Quantum Dots
... temperatures (eVbias , kB T << e2 /CΣ ). In Fig. 3(a) transport through the dot is blocked due to the Coulomb blockade effect, with N electrons on the dot. By decreasing the gate voltage, the chemical potential µN inside the dot [equation (6)] is raised until it aligns with that of the drain contact ...
... temperatures (eVbias , kB T << e2 /CΣ ). In Fig. 3(a) transport through the dot is blocked due to the Coulomb blockade effect, with N electrons on the dot. By decreasing the gate voltage, the chemical potential µN inside the dot [equation (6)] is raised until it aligns with that of the drain contact ...
Landau Levels in Two and Three-Dimensional Electron Gases in a
... The electrons within one Landau leavel may be considered to behave as if they were one-dimensional. The density of states (DOS), %(E ), which in the abstance of a magnetic ld is a parabola given by %(E ) / E 1 2 dE , now becomes the sum of a set of one-dimensional densities of states, where %(E ) / ...
... The electrons within one Landau leavel may be considered to behave as if they were one-dimensional. The density of states (DOS), %(E ), which in the abstance of a magnetic ld is a parabola given by %(E ) / E 1 2 dE , now becomes the sum of a set of one-dimensional densities of states, where %(E ) / ...
On the Stability of Classical Orbits of the Hydrogen Ground State in
... 2 h̄ω, where Planck’s constant is a constant of nature characterising the energy stored in these modes. The task is then to show that SED explains all quantum behaviour of matter at the statistical level. This has been argued to occur on general grounds [1,2]. SED is not ruled out by Bell’s theorem, ...
... 2 h̄ω, where Planck’s constant is a constant of nature characterising the energy stored in these modes. The task is then to show that SED explains all quantum behaviour of matter at the statistical level. This has been argued to occur on general grounds [1,2]. SED is not ruled out by Bell’s theorem, ...
H - Quantum Condensed Matter Research Group
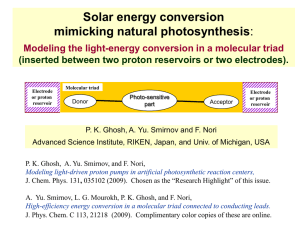
... J. Phys. Chem. C 113, 21218 (2009). Complimentary color copies of these are online. ...
... J. Phys. Chem. C 113, 21218 (2009). Complimentary color copies of these are online. ...
Wave properties of particles
... Not only light does have “schizophrenia”, so are other microscopic ``particle’’ such as electron, (see later chapters), i.e. particle” also manifest wave characteristics in some experiments Wave-particle duality is essentially the manifestation of the quantum nature of things This is an very we ...
... Not only light does have “schizophrenia”, so are other microscopic ``particle’’ such as electron, (see later chapters), i.e. particle” also manifest wave characteristics in some experiments Wave-particle duality is essentially the manifestation of the quantum nature of things This is an very we ...
chapter2.1
... 2. Identify the characteristics of protons, neutrons, and electrons. (Section 2.2; Exercises 2.10 and 2.12) 3. Use the concepts of atomic number and mass number to determine the number of subatomic particles in isotopes and to write correct symbols for isotopes. (Section 2.3; Exercises 2.16 and 2.22 ...
... 2. Identify the characteristics of protons, neutrons, and electrons. (Section 2.2; Exercises 2.10 and 2.12) 3. Use the concepts of atomic number and mass number to determine the number of subatomic particles in isotopes and to write correct symbols for isotopes. (Section 2.3; Exercises 2.16 and 2.22 ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.







![Including Nuclear Degrees of Freedom in a Lattice Hamiltonian, P. L. Hagelstein, I. U. Chaudhary, This paper has been accepted for publication in J. Cond. Mat. Nucl. Sci. and will be published soon. An earlier version was posted on the LANL ArXiV (/0401667 [cond-mat.other] 20 Jan 2012).](http://s1.studyres.com/store/data/008865544_1-7814cfb311674879bdeee17e5de71e9e-300x300.png)