
L 33 Modern Physics [1] Modern Physics
... • In the classical picture, the electrons in atoms orbit around the nucleus just as the planets orbit around the Sun. • However, the laws of mechanics and electromagnetism predict that an orbiting electron should continually radiate electromagnetic waves, and very quickly the electron would loose al ...
... • In the classical picture, the electrons in atoms orbit around the nucleus just as the planets orbit around the Sun. • However, the laws of mechanics and electromagnetism predict that an orbiting electron should continually radiate electromagnetic waves, and very quickly the electron would loose al ...
L 34 Modern Physics [1]
... • In the classical picture, the electrons in atoms orbit around the nucleus just as the planets orbit around the Sun. • However, the laws of mechanics and electromagnetism predict that an orbiting electron should continually radiate electromagnetic waves, and very quickly the electron would loose al ...
... • In the classical picture, the electrons in atoms orbit around the nucleus just as the planets orbit around the Sun. • However, the laws of mechanics and electromagnetism predict that an orbiting electron should continually radiate electromagnetic waves, and very quickly the electron would loose al ...
Chemistry - Unit 6 What do you need to know?? This chapter is on
... It was Einstein, in 1905, who deduced the basis of the photoelectric effect. I like an example that Dr. Blaber, at Florida State University uses: The Photoelectric effect as a carnival game: "A popular carnival game is where you are given a giant mallet and have to hit a pad on the ground. This send ...
... It was Einstein, in 1905, who deduced the basis of the photoelectric effect. I like an example that Dr. Blaber, at Florida State University uses: The Photoelectric effect as a carnival game: "A popular carnival game is where you are given a giant mallet and have to hit a pad on the ground. This send ...
Atomic quantum and nuclear
... His model includes both classical and non-classical ideas His model included an attempt to explain why the atom was stable ...
... His model includes both classical and non-classical ideas His model included an attempt to explain why the atom was stable ...
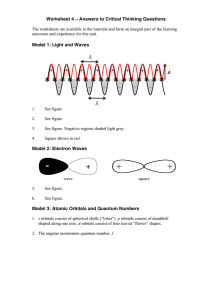
Answers to Critical Thinking Questions 4
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
... The 2s has one radial node and the 3s has two radial nodes. 3p have one radial node. In general, the number of radial nodes is equal to n – l - 1. ...
SpectraPart2
... Also see http://www.colorado.edu/physics/2000/quantumzone/ Recipe of stars and universe All that “glows” is 90% H and 10% He See last page for summary of spectra and electron transitions. ...
... Also see http://www.colorado.edu/physics/2000/quantumzone/ Recipe of stars and universe All that “glows” is 90% H and 10% He See last page for summary of spectra and electron transitions. ...
+l.
... 3-The Bohr Model of the Atom Bohr proposed that the possible energy states for atomic electrons were quantized – only certain values were possible. Then the spectrum could be explained as transitions from one level to another. ...
... 3-The Bohr Model of the Atom Bohr proposed that the possible energy states for atomic electrons were quantized – only certain values were possible. Then the spectrum could be explained as transitions from one level to another. ...
Views on Atomic Stru..
... Protons and Neutrons From Rutherford’s experiments, he was able to determine the amount of positive nuclear charge The positive charge was carried by particles called protons Scientists introduced the atomic number, which represents the number of protons in the nucleus of an atom James Chadwick dis ...
... Protons and Neutrons From Rutherford’s experiments, he was able to determine the amount of positive nuclear charge The positive charge was carried by particles called protons Scientists introduced the atomic number, which represents the number of protons in the nucleus of an atom James Chadwick dis ...
The Bohr Model of the Atom
... establish a new atomic model. Bohr made the following assumptions: In hydrogen atom 1. there can be only certain values of the total energy (electron's kinetic energy +potential energy). Quantized energy levels. 2. These allowed energy levels correspond to different orbits for the electron as it mov ...
... establish a new atomic model. Bohr made the following assumptions: In hydrogen atom 1. there can be only certain values of the total energy (electron's kinetic energy +potential energy). Quantized energy levels. 2. These allowed energy levels correspond to different orbits for the electron as it mov ...
Chapter 9: Chemical Quantities
... Properties of Light - wavelength, frequency and energy of light - electromagnetic spectrum and energy of light - spectrum and energy of visible light ...
... Properties of Light - wavelength, frequency and energy of light - electromagnetic spectrum and energy of light - spectrum and energy of visible light ...
L 34 Modern Physics [1]
... • In the classical picture, the electrons in atoms orbit around the nucleus just as the planets orbit around the Sun. • However, the laws of mechanics and electromagnetism predict that an orbiting electron should continually radiate electromagnetic waves, and very quickly the electron would loose al ...
... • In the classical picture, the electrons in atoms orbit around the nucleus just as the planets orbit around the Sun. • However, the laws of mechanics and electromagnetism predict that an orbiting electron should continually radiate electromagnetic waves, and very quickly the electron would loose al ...
Atomic Structure and Periodic Trends
... Use quantum numbers to describe orbitals. A given orbital can be described by a set of 3 quantum numbers: 1. Principal quantum number (n) 2. Angular momentum quantum number (l) 3. Magnetic quantum number (ml) ...
... Use quantum numbers to describe orbitals. A given orbital can be described by a set of 3 quantum numbers: 1. Principal quantum number (n) 2. Angular momentum quantum number (l) 3. Magnetic quantum number (ml) ...
Honors Chemistry Semester 1 Exam Review
... _________________________________________________________________________________________ 2. What is the overall charge of an atom? Why? _________________________________________________________ ______________________________________________________________________________________________ 3. Isotope ...
... _________________________________________________________________________________________ 2. What is the overall charge of an atom? Why? _________________________________________________________ ______________________________________________________________________________________________ 3. Isotope ...
Notes - Ms. Dawkins
... A neutron has about the ______________ ___________ as a proton. They are grouped together in the ______________________. Atoms are extremely ________________. The electron cloud is about _______________ times the size of the __________________. Electrons are much smaller than _____________________ ...
... A neutron has about the ______________ ___________ as a proton. They are grouped together in the ______________________. Atoms are extremely ________________. The electron cloud is about _______________ times the size of the __________________. Electrons are much smaller than _____________________ ...
Midterm Review
... • A strontium atom differs from a strontium ion in that the atom has a greater 1. number of electrons 2 number of protons ...
... • A strontium atom differs from a strontium ion in that the atom has a greater 1. number of electrons 2 number of protons ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.
![L 33 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003217156_1-265c5a519e2bca3f33717b4abd842898-300x300.png)
![L 34 Modern Physics [1]](http://s1.studyres.com/store/data/008622077_1-047a8df5b8f51427a7d951942e25e95f-300x300.png)













![L 34 Modern Physics [1]](http://s1.studyres.com/store/data/001537103_1-dca58a96feb57d01fab60ba8bdd791ec-300x300.png)






