
Does the Everyday World Really Obey Quantum Mechanics?
... PB or C = PB + PC + 2ABAC Suppose AC = ±AB, at random. Then average of PB or C is av. of AB AC PB or C = PB + PC + 2ABAC but ABAC = av. of +A2B and -A2B = 0 so PB or C =PB + PC “COMMON SENSE” RESULT, i.e.“as if” each system chose path B or path C WHEN AB AND AC SIMULTANEOUSLY “EXIST”, NEITHER B N ...
... PB or C = PB + PC + 2ABAC Suppose AC = ±AB, at random. Then average of PB or C is av. of AB AC PB or C = PB + PC + 2ABAC but ABAC = av. of +A2B and -A2B = 0 so PB or C =PB + PC “COMMON SENSE” RESULT, i.e.“as if” each system chose path B or path C WHEN AB AND AC SIMULTANEOUSLY “EXIST”, NEITHER B N ...
Untitled - Washington County Schools
... particle. Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. Charges are also found in tiny particles of matter. The electron always has a "-", or negative, charge. The proton always has a "+", or positive, charge. If ...
... particle. Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. Charges are also found in tiny particles of matter. The electron always has a "-", or negative, charge. The proton always has a "+", or positive, charge. If ...
electromagnetic spectrum and flame tests
... Let’s Review Energy – Radiation of different wavelengths affect matter differently – certain wavelengths (near infrared) may burn your skin with a heat burn, overexposure to X radiation causes tissue damage. These diverse effects are due to differences in the energy of the radiation. Radiation of h ...
... Let’s Review Energy – Radiation of different wavelengths affect matter differently – certain wavelengths (near infrared) may burn your skin with a heat burn, overexposure to X radiation causes tissue damage. These diverse effects are due to differences in the energy of the radiation. Radiation of h ...
Unit 2: Atom - newshamchemistry
... a. Plum pudding model/ seeds in a watermelon b. electron Robert Millikan a. electron What two inferences were made about the atomic structure at the end of the 19th century beginning of the 20th century? Rutherford a. Gold foil experiment b. Nucleus c. Alpha particles d. Copy figure 6 & 7 along with ...
... a. Plum pudding model/ seeds in a watermelon b. electron Robert Millikan a. electron What two inferences were made about the atomic structure at the end of the 19th century beginning of the 20th century? Rutherford a. Gold foil experiment b. Nucleus c. Alpha particles d. Copy figure 6 & 7 along with ...
Arrangement of Electrons in Atoms
... For a given metal, no electrons were emitted if the lights frequency was below a certain minimum. The brightness of a light won’t necessarily cause electrons to flow. Ex. Red light will not cause electrons to flow in a sheet of sodium metal, no matter how long or bright the source is. Violet light w ...
... For a given metal, no electrons were emitted if the lights frequency was below a certain minimum. The brightness of a light won’t necessarily cause electrons to flow. Ex. Red light will not cause electrons to flow in a sheet of sodium metal, no matter how long or bright the source is. Violet light w ...
Exam Study Questions for Quantum Effects
... Why do atoms emit light? Why do atoms emit light at very specific frequencies? What is the Bohr Atom, what did it explain? What two forces are equated to analyze the Bohr atom? What physical quantity did Bohr quantize (limit to specific values)? Why did the Bohr atom not predict? What fundamental eq ...
... Why do atoms emit light? Why do atoms emit light at very specific frequencies? What is the Bohr Atom, what did it explain? What two forces are equated to analyze the Bohr atom? What physical quantity did Bohr quantize (limit to specific values)? Why did the Bohr atom not predict? What fundamental eq ...
Chapter 6 lecture 1
... Bohr proposed that: the electron moves around the proton only in circular 'orbits' of certain allowed radii, which correspond to certain definite energies ...
... Bohr proposed that: the electron moves around the proton only in circular 'orbits' of certain allowed radii, which correspond to certain definite energies ...
for the p sublevel
... orbits have different shapes and the orbits could tilt in the presence of a magnetic field. Orbits can appear circular or elliptical, and they can even swing back and forth through the nucleus in a straight line. ...
... orbits have different shapes and the orbits could tilt in the presence of a magnetic field. Orbits can appear circular or elliptical, and they can even swing back and forth through the nucleus in a straight line. ...
Unit 1, Lecture 1
... the other side inside a vacuum tube after an electrical potential is created. By measuring the deflection in an electrical field he could show that the beam consisted of negatively charged particles ...
... the other side inside a vacuum tube after an electrical potential is created. By measuring the deflection in an electrical field he could show that the beam consisted of negatively charged particles ...
Student Text, pp. 650-653
... The equations in Section 12.5 suggest that we can be very precise about the properties of an electron in the hydrogen atom. We can determine an orbiting electron’s exact location using the equation for rn and can likewise determine its exact speed and energy. In 1927, the German physicist Werner Hei ...
... The equations in Section 12.5 suggest that we can be very precise about the properties of an electron in the hydrogen atom. We can determine an orbiting electron’s exact location using the equation for rn and can likewise determine its exact speed and energy. In 1927, the German physicist Werner Hei ...
electrons - RoncalliPhysics
... • There exists a certain minimum frequency of incident radiation below which no photoelectrons can be emitted. This frequency is called the threshold frequency. • Increase in intensity of incident beam increases the the photoelectric current, though stopping voltage remains the same. It does not cha ...
... • There exists a certain minimum frequency of incident radiation below which no photoelectrons can be emitted. This frequency is called the threshold frequency. • Increase in intensity of incident beam increases the the photoelectric current, though stopping voltage remains the same. It does not cha ...
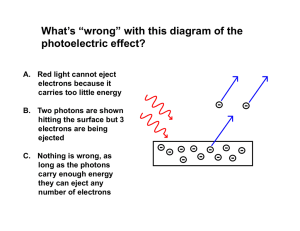
L 35 Modern Physics [1] Modern Physics
... • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
Exam 1 Topics to Review (McMurry Chpts 1
... 8. Homogeneous or heterogeneous mixture?: a) salsa b) gasoline 9. Is H2 an element or a compound? An atom? A molecule? 10. What is the volume of liquid in this graduated cylinder? ...
... 8. Homogeneous or heterogeneous mixture?: a) salsa b) gasoline 9. Is H2 an element or a compound? An atom? A molecule? 10. What is the volume of liquid in this graduated cylinder? ...
Define:
... 43. In the number 0.305 L, which digit is estimated? 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi ...
... 43. In the number 0.305 L, which digit is estimated? 44. Express the sum of 8.67 m and 5.2 m to the correct number of significant figures. 45. Express the product of 5.5 mm and 2.00 mm to the correct number of significant figures. 46. List the metric prefixes and their decimal equivalents. Ex: centi ...
The Bohr Theory, Matter Waves, and Quantum Theory
... Here, light impinges on a surface and electrons may be emitted. Classically, the energy of the light is controlled by the intensity of the light. Energy conservation products that there should be no dependence on wavelength of light. If the intensity of the light is increased, the kinetic energy of ...
... Here, light impinges on a surface and electrons may be emitted. Classically, the energy of the light is controlled by the intensity of the light. Energy conservation products that there should be no dependence on wavelength of light. If the intensity of the light is increased, the kinetic energy of ...
Light problems
... 6.____ _____ are the most penetrating kind of electromagnetic radiation. a. Radio waves b. Microwaves c. Gamma rays ...
... 6.____ _____ are the most penetrating kind of electromagnetic radiation. a. Radio waves b. Microwaves c. Gamma rays ...
For a “black body” - The University of Sheffield
... •Oscillator only allowed to have energy in integer multiples of some constant times the oscillator frequency: E = nhf •Average energy of oscillator at temperature T: ...
... •Oscillator only allowed to have energy in integer multiples of some constant times the oscillator frequency: E = nhf •Average energy of oscillator at temperature T: ...
1. Larger a
... hydrogen, contain more than a single electron, and those electrons interact with one another, in addition to interacting with the positively charged atomic nucleus; (2) It turns out that further quantum numbers (in addition to the number n which we encountered for hydrogen) restrict the allowed ener ...
... hydrogen, contain more than a single electron, and those electrons interact with one another, in addition to interacting with the positively charged atomic nucleus; (2) It turns out that further quantum numbers (in addition to the number n which we encountered for hydrogen) restrict the allowed ener ...
Atomic Structure and Periodicity
... 14. Which group possesses the lowest first ionization energy in their respective period? For 15 – 17: a. Bohr model b. deBroglie’s wave hypothesis c. Heisenberg’s uncertainty principle d. Quantum theory e. Atomic theory 15. Which principle provides that all matter may be considered a wave? 16. What ...
... 14. Which group possesses the lowest first ionization energy in their respective period? For 15 – 17: a. Bohr model b. deBroglie’s wave hypothesis c. Heisenberg’s uncertainty principle d. Quantum theory e. Atomic theory 15. Which principle provides that all matter may be considered a wave? 16. What ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.












![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003926344_1-b779c05b753c6dc3972377c21f9bdcd3-300x300.png)









