Atoms, Molecules, and Ions C Kapler ` , , I 27 O//#W SELF
... 10. A nuclear symbol for the element simultaneously in group 5 and period 4 is a 40 ...
... 10. A nuclear symbol for the element simultaneously in group 5 and period 4 is a 40 ...
Lecture-3: Atomic Structure
... and drop back to lower energy levels. • This process of dropping back, energy is emitted in the form of line spectrum containing various lines of particular frequency and wavelength. ...
... and drop back to lower energy levels. • This process of dropping back, energy is emitted in the form of line spectrum containing various lines of particular frequency and wavelength. ...
Electrons in a Shell - University of California, Berkeley
... It is interesting to examine the range of values of N where this solution is valid. First, we find the critical number of electrons N* at which the non-relativistic approximation used to obtain this result breaks down. This occurs when the Fermi ...
... It is interesting to examine the range of values of N where this solution is valid. First, we find the critical number of electrons N* at which the non-relativistic approximation used to obtain this result breaks down. This occurs when the Fermi ...
Quantum Mechanics in a Nutshell
... atoms are composed of positively charged nuclei surrounded by a cloud of “orbiting” electrons. But, – Orbiting (or accelerating) charge radiates energy electrons should spiral into nucleus all of matter should be unstable! – Spectroscopy results of H (Rydberg states) indicated that energy of an ...
... atoms are composed of positively charged nuclei surrounded by a cloud of “orbiting” electrons. But, – Orbiting (or accelerating) charge radiates energy electrons should spiral into nucleus all of matter should be unstable! – Spectroscopy results of H (Rydberg states) indicated that energy of an ...
Solid - burgess
... find an electron. 3. Subatomic particles a. nucleus-center region; positively charged; contains most of the atom’s mass Video Clip i. proton –positive particle ii. neutron-neutral particle (no charge) b. electron cloud i. outer region of the atom; mostly empty space ii. electron-the negative particl ...
... find an electron. 3. Subatomic particles a. nucleus-center region; positively charged; contains most of the atom’s mass Video Clip i. proton –positive particle ii. neutron-neutral particle (no charge) b. electron cloud i. outer region of the atom; mostly empty space ii. electron-the negative particl ...
L 35 Modern Physics [1]
... Niels Bohr, a Danish physicist, used the quantum concept to explain the nature of the atom. Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy If this were so, then we would observe that atoms emit light over a continuous range of ...
... Niels Bohr, a Danish physicist, used the quantum concept to explain the nature of the atom. Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy If this were so, then we would observe that atoms emit light over a continuous range of ...
Atomic Spectra - Northeast High School
... With less energy the electron orbitals should shrink and the electrons should spiral into the nucleus ...
... With less energy the electron orbitals should shrink and the electrons should spiral into the nucleus ...
Chapter 5
... • This idea agreed very well with Bohr's idea of quantized energy levels: only certain energies and therefore, wavelengths would be allowed in the atom. • This explained why only certain colors (wavelengths) were seen in the spectrum of the hydrogen atom. ...
... • This idea agreed very well with Bohr's idea of quantized energy levels: only certain energies and therefore, wavelengths would be allowed in the atom. • This explained why only certain colors (wavelengths) were seen in the spectrum of the hydrogen atom. ...
Chapter 5
... • This idea agreed very well with Bohr's idea of quantized energy levels: only certain energies and therefore, wavelengths would be allowed in the atom. • This explained why only certain colors (wavelengths) were seen in the spectrum of the hydrogen atom. ...
... • This idea agreed very well with Bohr's idea of quantized energy levels: only certain energies and therefore, wavelengths would be allowed in the atom. • This explained why only certain colors (wavelengths) were seen in the spectrum of the hydrogen atom. ...
Science Olympiad
... ______ 7. In moving from left to right across a period in the periodic table of the elements (A) ionization energy decreases due to increases shielding effect. (B) atomic radius decreases due to an increase in effective nuclear charge. (C) electronegativity decreases due to an increase in atomic rad ...
... ______ 7. In moving from left to right across a period in the periodic table of the elements (A) ionization energy decreases due to increases shielding effect. (B) atomic radius decreases due to an increase in effective nuclear charge. (C) electronegativity decreases due to an increase in atomic rad ...
Abstract - Quantum Realism and Special Reference
... if classically interpreted) are unstable, since accelerated charges radiate energy. It is interesting that Bohr [1913/1967, p. 141] also cites one piece of empirical evidence here, that being that in a high vacuum more spectral lines of the Balmer series for hydrogen are apparent. To the best of my ...
... if classically interpreted) are unstable, since accelerated charges radiate energy. It is interesting that Bohr [1913/1967, p. 141] also cites one piece of empirical evidence here, that being that in a high vacuum more spectral lines of the Balmer series for hydrogen are apparent. To the best of my ...
Chapter 4 - SchoolRack
... Rutherford’s model did not explain where electrons were – what prevented electrons from being drawn into nucleus? New model arose from experiments involving absorption and emission of light by matter ...
... Rutherford’s model did not explain where electrons were – what prevented electrons from being drawn into nucleus? New model arose from experiments involving absorption and emission of light by matter ...
Chemistry CP Final Exam Review #2
... Define the following terms: electromagnetic radiation, wavelength, frequency, photons, quantized, line spectrum, continuous spectrum, orbital, principal energy levels, sublevels, electron configuration, orbital diagram, valence electrons, core electrons, representative elements, metals, nonmetals, m ...
... Define the following terms: electromagnetic radiation, wavelength, frequency, photons, quantized, line spectrum, continuous spectrum, orbital, principal energy levels, sublevels, electron configuration, orbital diagram, valence electrons, core electrons, representative elements, metals, nonmetals, m ...
Quiz 4
... 4. (7 points) An electron in a certain atom is in the n = 2 quantum level. List the possible values of l (and for each l list all values of ml ) that it can have. The angular momentum quantum number l can have integral (i.e. whole number) values from 0 to n − 1. In this case n = 2, so the allowed va ...
... 4. (7 points) An electron in a certain atom is in the n = 2 quantum level. List the possible values of l (and for each l list all values of ml ) that it can have. The angular momentum quantum number l can have integral (i.e. whole number) values from 0 to n − 1. In this case n = 2, so the allowed va ...
10 ≥ t 137 ≈ e cħ He re − mp vm E 2 2 1
... We thus find that in singly charged helium atom the electron circulate around the nucleus with a velocity twice as large as in case of hydrogen atom. When we analyse Eq. (10) and Eq. (11), we see that as the nuclear charge increases from Z = 1 to Z = 2, the electron orbits in hydrogen like atom come ...
... We thus find that in singly charged helium atom the electron circulate around the nucleus with a velocity twice as large as in case of hydrogen atom. When we analyse Eq. (10) and Eq. (11), we see that as the nuclear charge increases from Z = 1 to Z = 2, the electron orbits in hydrogen like atom come ...
Notes
... before going onto a different type of room. 4. When filling rooms on a floor, you must place one student in each type of room before pairing them. ...
... before going onto a different type of room. 4. When filling rooms on a floor, you must place one student in each type of room before pairing them. ...
Worksheet Key - UCSB C.L.A.S.
... Wave C is the longest so it has the lowest energy ⇒ n=4 → n=3 Wave B is the second longest so second lowest energy ⇒ n=5 → n=3 Wave A is the third longest so third lowest energy ⇒ n=6 → n=3 b. If the wavelength of line B is 142.5 nm, calculate the wavelength of line A (nm). ...
... Wave C is the longest so it has the lowest energy ⇒ n=4 → n=3 Wave B is the second longest so second lowest energy ⇒ n=5 → n=3 Wave A is the third longest so third lowest energy ⇒ n=6 → n=3 b. If the wavelength of line B is 142.5 nm, calculate the wavelength of line A (nm). ...
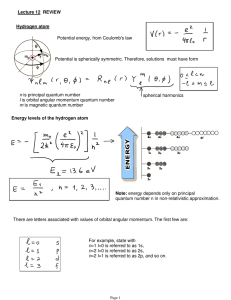
Lecture 12: Review.
... Example: hydrogen ground state J=1/2, I=1/2 (proton) F=0,1, so two levels (see picture on previous page) ...
... Example: hydrogen ground state J=1/2, I=1/2 (proton) F=0,1, so two levels (see picture on previous page) ...
PHYS 481/681 Quantum Mechanics Stephen Lepp August 29, 2016
... Introduction to Quantum Mechanics nd the interpretation of its solutions, the uncertainty principles, one-dimensional problems, harmonic oscillator, angular momentum, the hydrogen atom. 3 credits. • Class MW 11:30-12:45 BPB 249. • Office Hours TTh 12:45-1:30 or by arrangement. • Textbook “Quantum Me ...
... Introduction to Quantum Mechanics nd the interpretation of its solutions, the uncertainty principles, one-dimensional problems, harmonic oscillator, angular momentum, the hydrogen atom. 3 credits. • Class MW 11:30-12:45 BPB 249. • Office Hours TTh 12:45-1:30 or by arrangement. • Textbook “Quantum Me ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.





![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/001036078_1-1a4f17b9367db590f7dcb987ef21bbe6-300x300.png)