Early Atomic Models – From Mechanical to Quantum
... ignorance of their basic nature, Rutherford gradually increased the complexity of the experimental questions he posed. Are the α-‐rays deflected by a magnetic field? Are the α-‐particles positivel ...
... ignorance of their basic nature, Rutherford gradually increased the complexity of the experimental questions he posed. Are the α-‐rays deflected by a magnetic field? Are the α-‐particles positivel ...
Chapter 6 Electronic Structure of Atoms
... ____________________ of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of ...
... ____________________ of the orbital. • Allowed values of l are integers ranging from 0 to n − 1. • We use letter designations to communicate the different values of l and, therefore, the shapes and types of ...
Section 7.5 Quantum Mechanics and the Atom
... Light of three different wavelengths 325 nm, 455 nm and 632 nm shines on a metal surface. Match the wavelength with the ...
... Light of three different wavelengths 325 nm, 455 nm and 632 nm shines on a metal surface. Match the wavelength with the ...
Question, hints, and answers. Look at hints if you need help. Look at
... Molecules in a sample of NH3(l) are held closely together by intermolecular forces *hint In the NH3 molecule, there is a covalent bond between N and H. But the N "wants" the electrons more than the H does, so it pulls them closer to itself. You end up with a little more than half the negative charge ...
... Molecules in a sample of NH3(l) are held closely together by intermolecular forces *hint In the NH3 molecule, there is a covalent bond between N and H. But the N "wants" the electrons more than the H does, so it pulls them closer to itself. You end up with a little more than half the negative charge ...
Advanced Quantum Physics - Theory of Condensed Matter
... Although no rigorous derivation, Schrödinger’s equation can be motivated by developing connection between light waves and photons, and constructing analogous structure for de Broglie waves and electrons. For a monochromatic wave in vacuo, Maxwell’s wave equation, ...
... Although no rigorous derivation, Schrödinger’s equation can be motivated by developing connection between light waves and photons, and constructing analogous structure for de Broglie waves and electrons. For a monochromatic wave in vacuo, Maxwell’s wave equation, ...
Atoms
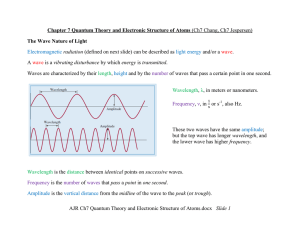
... You have learned that a light beam consists of photons. The intensity of a beam is proportional to the number of photons. The distribution of intensity versus frequency (or wavelength) is a spectrum. A white light consists of photons with all frequencies in the visible region, and it has a continuou ...
... You have learned that a light beam consists of photons. The intensity of a beam is proportional to the number of photons. The distribution of intensity versus frequency (or wavelength) is a spectrum. A white light consists of photons with all frequencies in the visible region, and it has a continuou ...
Problems
... the conduction electrons in the metal. Here is our mode of attack: when the light wave experiences a mismatch in the refractive index, part of it is transmitted, and part reflected. So we will ask Maxwell’s equations to give us the reflection coefficient when a light beam is incident from air on a m ...
... the conduction electrons in the metal. Here is our mode of attack: when the light wave experiences a mismatch in the refractive index, part of it is transmitted, and part reflected. So we will ask Maxwell’s equations to give us the reflection coefficient when a light beam is incident from air on a m ...
30.09.2013 1 Chapter 2 Atoms and Molecules Warning!! Chapter
... • Polymers are very large molecules made up of many smaller molecules linked together. • Monomers - The small molecules linked together in ...
... • Polymers are very large molecules made up of many smaller molecules linked together. • Monomers - The small molecules linked together in ...
PHYS 1443 – Section 501 Lecture #1
... – Must be treated quantum mechanically via • probability distributions or expectation values • Atomic size is the average coordinate of the outermost electron and calculable via QM using Coulomb potential • Not calculable for nucleus since the potential is not known – Must rely on experimental measu ...
... – Must be treated quantum mechanically via • probability distributions or expectation values • Atomic size is the average coordinate of the outermost electron and calculable via QM using Coulomb potential • Not calculable for nucleus since the potential is not known – Must rely on experimental measu ...
Physical Chemistry II – Exam 3 Solutions
... Note that this expectation value makes sense, because it corresponds to the Coulomb potential of interaction between the nucleus and an electron at a distance a0 , which is the ...
... Note that this expectation value makes sense, because it corresponds to the Coulomb potential of interaction between the nucleus and an electron at a distance a0 , which is the ...
Quantum Theory
... Quantum Theory, in physics, description of the particles that make up matter and how they interact with each other and with energy. Quantum theory explains in principle how to calculate what will happen in any experiment involving physical or biological systems, and how to understand how our world w ...
... Quantum Theory, in physics, description of the particles that make up matter and how they interact with each other and with energy. Quantum theory explains in principle how to calculate what will happen in any experiment involving physical or biological systems, and how to understand how our world w ...
Quantum Theory
... in the measurement of a particle’s position and the uncertainty in the measurement of its momentum. Heisenberg said that the uncertainty in position (represented by Δx) times the uncertainty in momentum (represented by Δp;) must be greater than a constant number equal to Planck’s constant (h) divide ...
... in the measurement of a particle’s position and the uncertainty in the measurement of its momentum. Heisenberg said that the uncertainty in position (represented by Δx) times the uncertainty in momentum (represented by Δp;) must be greater than a constant number equal to Planck’s constant (h) divide ...
Testing a Mechanical Behavior of Light
... The results indicate a pattern relatively similar to the experimental results ...
... The results indicate a pattern relatively similar to the experimental results ...
Quantum Entanglements and Hauntological Relations of Inheritance
... Prize for his ‘crazy idea’ of the photon (light quantum), not for relativity. Bohr’s idea: The nucleus remains at the atom’s center, but electrons don’t orbit the nucleus (pace Rutherford). Rather, each electron resides in one of a finite set of discrete/quantised energy levels, and atoms only emit ...
... Prize for his ‘crazy idea’ of the photon (light quantum), not for relativity. Bohr’s idea: The nucleus remains at the atom’s center, but electrons don’t orbit the nucleus (pace Rutherford). Rather, each electron resides in one of a finite set of discrete/quantised energy levels, and atoms only emit ...
Atomic Structure - The Student Room
... All group 1,2 and 3 elements have relatively low ionisation energies, although there is a large jump in all atoms when another shell is entered when the electrons in the most outer shell are all lost. The next electron is closer to the nucleus so atomic radius and electron shielding decrease, so nuc ...
... All group 1,2 and 3 elements have relatively low ionisation energies, although there is a large jump in all atoms when another shell is entered when the electrons in the most outer shell are all lost. The next electron is closer to the nucleus so atomic radius and electron shielding decrease, so nuc ...
AJR Ch7 Quantum Theory and Electronic Structure of Atoms.docx
... According to classical electromagnetic (wave) theory, this effect can be attributed to the transfer of energy from the light to an electron. An alteration in either the intensity or wavelength of light would induce changes in the rate of emission of electrons from the metal. Furthermore, according ...
... According to classical electromagnetic (wave) theory, this effect can be attributed to the transfer of energy from the light to an electron. An alteration in either the intensity or wavelength of light would induce changes in the rate of emission of electrons from the metal. Furthermore, according ...
File
... 3. A scale ranking the desire for electrons, with nonmetals having the highest values. _______ 4. The number of protons in the nucleus of an atom. _______ 5. Atom of an element that has a specific number of protons and neutrons. _______ 6. Scientist who discovered that electrons must reside in fixed ...
... 3. A scale ranking the desire for electrons, with nonmetals having the highest values. _______ 4. The number of protons in the nucleus of an atom. _______ 5. Atom of an element that has a specific number of protons and neutrons. _______ 6. Scientist who discovered that electrons must reside in fixed ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.