Electronic Properties of Metals
... For homework you will show that using the classical kinetic theory of gases to express v gives a result that does not agree with experiment. What’s wrong? Consider once again the manifold of energy levels occupied by the FEG: ...
... For homework you will show that using the classical kinetic theory of gases to express v gives a result that does not agree with experiment. What’s wrong? Consider once again the manifold of energy levels occupied by the FEG: ...
The de Broglie wavelength is inversely proportional to
... De Broglie's key realization was that in a one-electron atom, for a wave to have a stable amplitude and not decay over time, aninteger number (n) of wavelengths must fit into a single circumference drawn by the Bohr orbit. He related this to the principal quantum number n through the equation: ...
... De Broglie's key realization was that in a one-electron atom, for a wave to have a stable amplitude and not decay over time, aninteger number (n) of wavelengths must fit into a single circumference drawn by the Bohr orbit. He related this to the principal quantum number n through the equation: ...
Walker3_Lecture_Ch30
... the temperature. This means that the temperature of a blackbody can be determined by its color. ...
... the temperature. This means that the temperature of a blackbody can be determined by its color. ...
Nondispersing Bohr Wave Packets - Physics (APS)
... the electron’s motion is not phase-locked to the MW field there is no variation in the signal, but if the atom has become a NWP there is a variation with the 56-ps period of the MW field [16,18]. The Fabry-Pérot cavity is composed of two 82-mmdiameter brass mirrors of 102-mm radius of curvature wit ...
... the electron’s motion is not phase-locked to the MW field there is no variation in the signal, but if the atom has become a NWP there is a variation with the 56-ps period of the MW field [16,18]. The Fabry-Pérot cavity is composed of two 82-mmdiameter brass mirrors of 102-mm radius of curvature wit ...
Chapter 7 - Moore Public Schools
... • The nuclear model of the atom does not explain what structural changes occur when the atom gains or loses energy. • Bohr developed a model of the atom to explain how the structure of the atom changes when it undergoes energy transitions. • Bohr’s major idea was that the energy of the atom was quan ...
... • The nuclear model of the atom does not explain what structural changes occur when the atom gains or loses energy. • Bohr developed a model of the atom to explain how the structure of the atom changes when it undergoes energy transitions. • Bohr’s major idea was that the energy of the atom was quan ...
Chapter 2 (Hill/Petrucci/McCreary/Perry This chapter deals with
... 1. all matter is composed of small, invisible particles called atoms 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involv ...
... 1. all matter is composed of small, invisible particles called atoms 2. in chemical reactions, atoms are neither created nor destroyed 3. atoms of each element have unique properties - all atoms of a given atom are identical and have identical masses and other properties 4. chemical reactions involv ...
1 Handout #11 ME 262A Summary on Quantum States We showed
... the overall Schrödinger equation is factored into. Note that the energy states can be degenerate (i.e., there can be many combinations of nj that give rise to the same values of translational energy). Often, we speak of energy levels, and introduce the level degeneracy, gtr , as the number of possib ...
... the overall Schrödinger equation is factored into. Note that the energy states can be degenerate (i.e., there can be many combinations of nj that give rise to the same values of translational energy). Often, we speak of energy levels, and introduce the level degeneracy, gtr , as the number of possib ...
Physical Chemistry - School of Chemistry, University of Leeds
... To help with the assignment, consider the following questions: which transition in the Balmer series has the lowest energy change? which of the observed lines corresponds to the lowest energy? In this way, assign the principal quantum number n2 of the excited state to each of the observed lines ...
... To help with the assignment, consider the following questions: which transition in the Balmer series has the lowest energy change? which of the observed lines corresponds to the lowest energy? In this way, assign the principal quantum number n2 of the excited state to each of the observed lines ...
symmetry in atomic and molecular systems
... constant is the product of the charges carried by the particles. This potential energy has exactly the same form as that of the gravitational attraction between two masses; only the constant c changes to the product of the two masses. The helium atom consists of a nucleus with a positive charge of ÷ ...
... constant is the product of the charges carried by the particles. This potential energy has exactly the same form as that of the gravitational attraction between two masses; only the constant c changes to the product of the two masses. The helium atom consists of a nucleus with a positive charge of ÷ ...
Quantum energy distribution function of hot electrons in
... general a direct solution of (14) is impossible. happens that p.(s) varies slowly on the scale (8’ 8) of energy transfers, one can transform (14) into a differential equation of the Fokker-Planck type, thereby gaining more insight; we shall first explore this limiting case without specifying the nat ...
... general a direct solution of (14) is impossible. happens that p.(s) varies slowly on the scale (8’ 8) of energy transfers, one can transform (14) into a differential equation of the Fokker-Planck type, thereby gaining more insight; we shall first explore this limiting case without specifying the nat ...
Chapter 12 Multiple Particle States
... function should collapse when that particle is measured. This variable is “hidden” because it is not accounted for in quantum mechanics. In the early 1960’s, physicist John Bell proposed experiments that would test the EPR paradox by being able to tell the difference between the standard predictions ...
... function should collapse when that particle is measured. This variable is “hidden” because it is not accounted for in quantum mechanics. In the early 1960’s, physicist John Bell proposed experiments that would test the EPR paradox by being able to tell the difference between the standard predictions ...
Atomic Structure and the Periodic Table
... A circle is used to represent an electron orbital within an energy sublevel. Notice that the energy of an electron increases with an increasing value of the principal quantum number, n. For a given value of n, the sublevels increase in energy, in order, s
... A circle is used to represent an electron orbital within an energy sublevel. Notice that the energy of an electron increases with an increasing value of the principal quantum number, n. For a given value of n, the sublevels increase in energy, in order, s
投影片 1
... Intermediate particles with similar masses were discovered and named as π mesons. In quantum field theory, forces between particles are described by the exchange of virtual particles: p → p + π0 → p , ...
... Intermediate particles with similar masses were discovered and named as π mesons. In quantum field theory, forces between particles are described by the exchange of virtual particles: p → p + π0 → p , ...
PPT
... F is the minimum energy needed to strip an electron from the metal. F is defined as positive. Not all electrons will leave with the maximum kinetic energy (due to losses). ...
... F is the minimum energy needed to strip an electron from the metal. F is defined as positive. Not all electrons will leave with the maximum kinetic energy (due to losses). ...
Experimental and Theoretical Charge Density Analysis of a
... charge density of the molecule was determined from both experimentally and theoretically derived diffraction data. The stereochemistry and electron density topology of the sulfonium group was analyzed. To understand the chemical reactivity of the molecule, the electrostatic potential difference betwee ...
... charge density of the molecule was determined from both experimentally and theoretically derived diffraction data. The stereochemistry and electron density topology of the sulfonium group was analyzed. To understand the chemical reactivity of the molecule, the electrostatic potential difference betwee ...
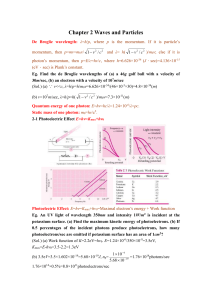
Chapter 2 Waves and Particles De Broglie wavelength: λ=h/p, where
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
... Eg. An UV light of wavelength 350nm and intensity 1W/m2 is incident at the potassium surface. (a) Find the maximum kinetic energy of photoelectrons. (b) If 0.5 percentages of the incident photons produce photoelectrons, how many photoelectrons/sec are emitted if potassium surface has an area of 1cm2 ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.