Precision Spectroscopy in Alkali Vapor
... atomic ensemble [5]. In this conventional configuration, the atomic vapor is illuminated by an optical source, resonant with the D1 or D2 transition between one of the hyperfine ground states and an absorbing optical state, as well as exposed to a magnetic field, oscillating at the hyperfine freque ...
... atomic ensemble [5]. In this conventional configuration, the atomic vapor is illuminated by an optical source, resonant with the D1 or D2 transition between one of the hyperfine ground states and an absorbing optical state, as well as exposed to a magnetic field, oscillating at the hyperfine freque ...
Early Atomic Models – From Mechanical to Quantum
... birth of the quantum atom in 1913. Beginning with almost no understanding of atoms other than their chemical and spectral properties, physicists were handed important clues to their i ...
... birth of the quantum atom in 1913. Beginning with almost no understanding of atoms other than their chemical and spectral properties, physicists were handed important clues to their i ...
The Quantum World
... classical physics I can know both where an electron is and what it is doing. In more technical language, its position and momentum can both simultaneously be known. Such an object is not so very different from a table or a cow, concerning which I can have similar information of where they are and wh ...
... classical physics I can know both where an electron is and what it is doing. In more technical language, its position and momentum can both simultaneously be known. Such an object is not so very different from a table or a cow, concerning which I can have similar information of where they are and wh ...
Ketterle lecture notes July 13th - Quantum Optics and Spectroscopy
... Time of flight (20 msec), standing wave excitation ...
... Time of flight (20 msec), standing wave excitation ...
Electronic and atomic structure of liquid potassium via
... where the second term in this equation corresponds to exchange between pairs of electrons, while the third term corresponds to a 3-cycle exchange or simultaneous exchange among three electrons, and so on. The difficulty in defining an effective potential resides in the fact that the partition functi ...
... where the second term in this equation corresponds to exchange between pairs of electrons, while the third term corresponds to a 3-cycle exchange or simultaneous exchange among three electrons, and so on. The difficulty in defining an effective potential resides in the fact that the partition functi ...
Quantum discreteness is an illusion
... field (see the remark concerning the “second quantization” in Sect. 2). So it may not be surprising that many scientists still believe that there is a wave function for each electron in an atom, or that the Dirac field equation was a relativistic generalization of the Schrödinger equation. According ...
... field (see the remark concerning the “second quantization” in Sect. 2). So it may not be surprising that many scientists still believe that there is a wave function for each electron in an atom, or that the Dirac field equation was a relativistic generalization of the Schrödinger equation. According ...
results, conjectures and applications to quasicrystals
... The framework we propose is valid within the one electron approximation. We will ignore explicitly the phonons and the electron–electron interactions as a first step. In this section only non-dissipative transport properties are considered. These are dominated by interference effects due to Bragg re ...
... The framework we propose is valid within the one electron approximation. We will ignore explicitly the phonons and the electron–electron interactions as a first step. In this section only non-dissipative transport properties are considered. These are dominated by interference effects due to Bragg re ...
Atomic structure and periodic table
... It can donate the three outer electrons to have stable electronic structure/configuration 2:8. It can gain five extra electrons to have stable electronic structure/configuration 2:8:8. Donating requires less energy, and thus Aluminium reacts by donating its three outer electrons. Elements with less ...
... It can donate the three outer electrons to have stable electronic structure/configuration 2:8. It can gain five extra electrons to have stable electronic structure/configuration 2:8:8. Donating requires less energy, and thus Aluminium reacts by donating its three outer electrons. Elements with less ...
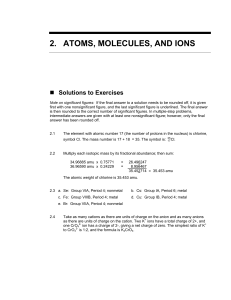
2.ATOMS, MOLECULES, AND IONS
... A mass spectrometer works by allowing a gas, such as neon, to pass through an inlet tube into a chamber where the atoms collide with electrons from an electron beam (cathode rays). The force of a collision can knock an electron from an atom. The positive neon atoms produced this way are drawn toward ...
... A mass spectrometer works by allowing a gas, such as neon, to pass through an inlet tube into a chamber where the atoms collide with electrons from an electron beam (cathode rays). The force of a collision can knock an electron from an atom. The positive neon atoms produced this way are drawn toward ...
quantum-gravity-presentation
... Quantum Gravity: Why so Difficult? • Don’t Buy the Tickets Quite Yet (III) • What Does it Mean to Have an Infinite Series with Terms of Increasing Dimension? • If You “Cutoff” the Series, You Can Apparently Fiddle with the Resulting Equations to Get Something With a Physical Meaning • But You Canno ...
... Quantum Gravity: Why so Difficult? • Don’t Buy the Tickets Quite Yet (III) • What Does it Mean to Have an Infinite Series with Terms of Increasing Dimension? • If You “Cutoff” the Series, You Can Apparently Fiddle with the Resulting Equations to Get Something With a Physical Meaning • But You Canno ...
Principles of Inorganic Chemistry Brochure
... - Coverage of atomic and molecular term symbols, symmetry coordinates in vibrational spectroscopy using the projection operator method, polyatomic MO theory, band theory, and Tanabe–Sugano diagrams - Worked examples throughout the text, unanswered problems in every chapter, and generous use of infor ...
... - Coverage of atomic and molecular term symbols, symmetry coordinates in vibrational spectroscopy using the projection operator method, polyatomic MO theory, band theory, and Tanabe–Sugano diagrams - Worked examples throughout the text, unanswered problems in every chapter, and generous use of infor ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).