Evade the Heisenberg Uncertainty Principle
... 2-D slice images. In a fundamental science laboratory, quantum state tomography is the process of completely characterizing the quantum state of an object as it is emitted by its source, before a possible measurement or interaction with the environment takes place. This technique has become an essen ...
... 2-D slice images. In a fundamental science laboratory, quantum state tomography is the process of completely characterizing the quantum state of an object as it is emitted by its source, before a possible measurement or interaction with the environment takes place. This technique has become an essen ...
File
... is like a map, telling you where an electron can be found in an atom. There are three rules that govern why an electron will be in one sublevel rather than another: 1.Pauli Exclusion Principle 2.Hund’s Rule 3.Aufbau Principle ...
... is like a map, telling you where an electron can be found in an atom. There are three rules that govern why an electron will be in one sublevel rather than another: 1.Pauli Exclusion Principle 2.Hund’s Rule 3.Aufbau Principle ...
2 - AQA
... The actual mass in grams of any atom or molecule is too tiny to find by weighing. Instead, the masses of atoms are compared and relative masses are used. This was done in the past by defining the relative atomic mass of hydrogen, the lightest element, as 1. The average mass of an atom of oxygen (for ...
... The actual mass in grams of any atom or molecule is too tiny to find by weighing. Instead, the masses of atoms are compared and relative masses are used. This was done in the past by defining the relative atomic mass of hydrogen, the lightest element, as 1. The average mass of an atom of oxygen (for ...
Elements, Compounds, and Chemical Equations
... 1. Counting Subatomic Particles: Use the periodic table • The atomic number is the same as the number of protons. • The number of protons is the same as the number of electrons. • The mass number is the protons added to the neutrons. ...
... 1. Counting Subatomic Particles: Use the periodic table • The atomic number is the same as the number of protons. • The number of protons is the same as the number of electrons. • The mass number is the protons added to the neutrons. ...
Paper
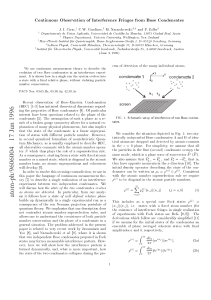
... element. This exchange term arises whenever the system occupies different quantum states which have spatial overlap @14#. g (2) (0)51 implies that the system can be described locally by a single wave function, but it does not rule out the population of numerous nonoverlapping quantum states. The fac ...
... element. This exchange term arises whenever the system occupies different quantum states which have spatial overlap @14#. g (2) (0)51 implies that the system can be described locally by a single wave function, but it does not rule out the population of numerous nonoverlapping quantum states. The fac ...
Voltage-tunable ferromagnetism in semimagnetic quantum dots with
... QDs can be stored not only in the number of carriers but also in the form of the Mn magnetization. Currently, magnetic QDs are a hot topic.17–26 One important property of Mndoped nanostructures is that a single particle 共electron or hole兲 can strongly alter the ground state of the system, leading to ...
... QDs can be stored not only in the number of carriers but also in the form of the Mn magnetization. Currently, magnetic QDs are a hot topic.17–26 One important property of Mndoped nanostructures is that a single particle 共electron or hole兲 can strongly alter the ground state of the system, leading to ...
Plotting Functions
... c. In the following columns, convert your data to radians and evaluate the trigonometric functions. (When Excel evaluates trig functions, it assumes that your data is in radians. However, since we’re used to dealing with things in degrees, we’ll start there.) d. Plot the following combinations of fu ...
... c. In the following columns, convert your data to radians and evaluate the trigonometric functions. (When Excel evaluates trig functions, it assumes that your data is in radians. However, since we’re used to dealing with things in degrees, we’ll start there.) d. Plot the following combinations of fu ...
The Influence of Retardation on the London
... decreases more rapidly than R '. Overbeek then pointed out that on the basis of the picture customarily used for visualizing London forces, an inHuence of retardation on the interaction is to be expected as soon as the distance between the particles becomes comparable to the wavelength corresponding ...
... decreases more rapidly than R '. Overbeek then pointed out that on the basis of the picture customarily used for visualizing London forces, an inHuence of retardation on the interaction is to be expected as soon as the distance between the particles becomes comparable to the wavelength corresponding ...
Hydrogen Storage in Magnesium Clusters
... shift in the lattice enthalpies can have a large impact on the desorption enthalpy. In general, the specific lattice enthalpy decreases upon lowering the amount of atoms in a cluster (Mgx or MgxH2x), due to the decreased average coordination number of the atoms. Distortions of the Mg(H2) lattice can ...
... shift in the lattice enthalpies can have a large impact on the desorption enthalpy. In general, the specific lattice enthalpy decreases upon lowering the amount of atoms in a cluster (Mgx or MgxH2x), due to the decreased average coordination number of the atoms. Distortions of the Mg(H2) lattice can ...
Separated spin-up and spin-down evolution of degenerated
... Increase of the discrete wave number kϕ = l/R gives an increase of whole dispersion surface. At the same time, the increase of l leads to increase of the shift ∆ξ(K, η). Form of surfaces describing the shift ∆ξ(K, η) also changes with increasing of l: area of small wave numbers and large spin polari ...
... Increase of the discrete wave number kϕ = l/R gives an increase of whole dispersion surface. At the same time, the increase of l leads to increase of the shift ∆ξ(K, η). Form of surfaces describing the shift ∆ξ(K, η) also changes with increasing of l: area of small wave numbers and large spin polari ...
Lie Groups and Quantum Mechanics
... The Lie bracket for this example turns out to be the familiar cross-product from vector algebra. (Unfortunately, I won’t get round to discussing the Lie bracket.) The Lie algebras of SO(3) and SU (2) are isomorphic. This is the chief technical justification for the “electron = spinning ball” analogy. ...
... The Lie bracket for this example turns out to be the familiar cross-product from vector algebra. (Unfortunately, I won’t get round to discussing the Lie bracket.) The Lie algebras of SO(3) and SU (2) are isomorphic. This is the chief technical justification for the “electron = spinning ball” analogy. ...
Lie Algebras and the Schr¨odinger equation: (quasi-exact-solvability, symmetric coordinates) Alexander Turbiner
... Radial parts of L-B operators ≡ Olshanetsky-Perelomov Hamiltonians relevant from physical point of view. They can be associated with root systems. ...
... Radial parts of L-B operators ≡ Olshanetsky-Perelomov Hamiltonians relevant from physical point of view. They can be associated with root systems. ...
Quantum Phase Transitions
... end result is seen in the action, which looks like that of a d + 1 Euclidean space-time integral, except that the extra temporal dimension is finite in extent (from 0 to β). As T → 0, we get the same (infinite) limits for a d + 1 effective classical system. This equivalent mapping between a d-dimens ...
... end result is seen in the action, which looks like that of a d + 1 Euclidean space-time integral, except that the extra temporal dimension is finite in extent (from 0 to β). As T → 0, we get the same (infinite) limits for a d + 1 effective classical system. This equivalent mapping between a d-dimens ...
Seeing a single photon without destroying it
... annihilate photons and convert them into electrical signals, making it impossible to see a single photon twice. But this limitation is not fundamentalÐquantum non-demolition strategies1±3 permit repeated measurements of physically observable quantities, yielding identical results. For example, quant ...
... annihilate photons and convert them into electrical signals, making it impossible to see a single photon twice. But this limitation is not fundamentalÐquantum non-demolition strategies1±3 permit repeated measurements of physically observable quantities, yielding identical results. For example, quant ...
ppt - Pavel Stránský
... - Stable x unstable trajectories - Poincaré sections: a manner of visualization - Fraction of regularity: a measure of chaos ...
... - Stable x unstable trajectories - Poincaré sections: a manner of visualization - Fraction of regularity: a measure of chaos ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).