FEYNMANWS PATH INTEGRAL APPROACH TO QUANTUM FIELD
... Here, Dirac’s S is the familiar action quantity L dt, where L(x; x; _ t) is the Lagrangian of classical mechanics. Now, how does this come about? The Lagrangian has everything built into it –kinetic and potential energy, including interaction terms –so if Dirac’s remark is true, then the propagator ...
... Here, Dirac’s S is the familiar action quantity L dt, where L(x; x; _ t) is the Lagrangian of classical mechanics. Now, how does this come about? The Lagrangian has everything built into it –kinetic and potential energy, including interaction terms –so if Dirac’s remark is true, then the propagator ...
Renormalization Group Seminar Exact solution to the Ising model
... construct explicitly the matrix in Fock space calculate the eigenvalues: ...
... construct explicitly the matrix in Fock space calculate the eigenvalues: ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 09. Give the relations which represent the Wiener-Khintchine theorem. 10. Write down the Boltzmann transport equation. PART – B Answer any FOUR questions. ...
... 09. Give the relations which represent the Wiener-Khintchine theorem. 10. Write down the Boltzmann transport equation. PART – B Answer any FOUR questions. ...
Fourth lecture, 28.10.03 (dispersion cancellation, time measurement
... Why? No interference between paths leading to different frequencies at the detectors, because in principle one could go back and measure how much energy had been absorbed. Note: it took a long time-integral to enforce this. If the detector had been open only for 1 fs, it would be impossible to tell ...
... Why? No interference between paths leading to different frequencies at the detectors, because in principle one could go back and measure how much energy had been absorbed. Note: it took a long time-integral to enforce this. If the detector had been open only for 1 fs, it would be impossible to tell ...
Potential Step: Griffiths Problem 2.33 Prelude: Note that the time
... This second-order differential equation is difficult to solve even for simple potentials encountered in classical mechanics, e.g., a charged particle in a constant electric field, V (x) = −qEx which leads to a constant force (i.e., constant acceleration, x = x0 + v0 t + (1/2)at2 and all that!) or th ...
... This second-order differential equation is difficult to solve even for simple potentials encountered in classical mechanics, e.g., a charged particle in a constant electric field, V (x) = −qEx which leads to a constant force (i.e., constant acceleration, x = x0 + v0 t + (1/2)at2 and all that!) or th ...
50 POINTS - University at Albany
... (a.) Consider a small but macroscopic particle of mass m = 1 micro-gram confined to a 1dimensional box with L = 1 micro-meter, e.g., a tiny bead on a very short wire. Compute the bead’s minimum kinetic energy. (b.) What are the corresponding momentum^2 and speed? (c.) Find the expectation value of t ...
... (a.) Consider a small but macroscopic particle of mass m = 1 micro-gram confined to a 1dimensional box with L = 1 micro-meter, e.g., a tiny bead on a very short wire. Compute the bead’s minimum kinetic energy. (b.) What are the corresponding momentum^2 and speed? (c.) Find the expectation value of t ...
Chapter 7 Relativistic Quantum Mechanics
... but quantum mechanically interactions can induce a jump to the negative branch releasing δE ≥ 2mc2 . Schrödinger hence arrived at his famous equation in the non-relativistic context. Two years later Paul A.M. Dirac found a linearization of the relativistic energy–momentum relation, which explained ...
... but quantum mechanically interactions can induce a jump to the negative branch releasing δE ≥ 2mc2 . Schrödinger hence arrived at his famous equation in the non-relativistic context. Two years later Paul A.M. Dirac found a linearization of the relativistic energy–momentum relation, which explained ...
Central potential
... system under study: different diatomic molecules have different reduced masses, or sizes. Note that the wave function of the system does not depend on r since we are neglecting the vibrations of the molecule. So the state of the quantum system is described by a wave function ψ(θ, φ) that depends only ...
... system under study: different diatomic molecules have different reduced masses, or sizes. Note that the wave function of the system does not depend on r since we are neglecting the vibrations of the molecule. So the state of the quantum system is described by a wave function ψ(θ, φ) that depends only ...
Chapter 28 - Purdue Physics
... theory): a cat, a flask of poison, and a radioactive source are placed in a sealed box. If an internal monitor detects radioactivity (i.e. a single atom decaying), the flask is shattered, releasing the poison that kills the cat. The Copenhagen interpretation of quantum mechanics implies that after a ...
... theory): a cat, a flask of poison, and a radioactive source are placed in a sealed box. If an internal monitor detects radioactivity (i.e. a single atom decaying), the flask is shattered, releasing the poison that kills the cat. The Copenhagen interpretation of quantum mechanics implies that after a ...
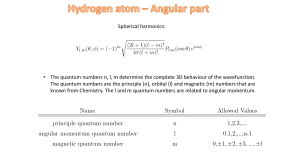
Spherical harmonics: • The quantum numbers n, l, m determine the
... • The quantum numbers n, l, m determine the complete 3D behaviour of the wavefunction. The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
... • The quantum numbers n, l, m determine the complete 3D behaviour of the wavefunction. The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
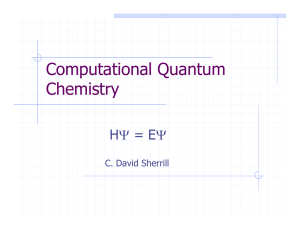
Computational Quantum Chemistry
... empirical parameters: applicable in principle to any molecule Quantum mechanics provides all information that can be knowable about a system (QM postulate). Often much more accurate and reliable. Computations can be vastly more timeconsuming. ...
... empirical parameters: applicable in principle to any molecule Quantum mechanics provides all information that can be knowable about a system (QM postulate). Often much more accurate and reliable. Computations can be vastly more timeconsuming. ...
WinFinal
... (f) If the magnetic field B in the deflection region doubles, how will the RADIUS of curvature of the particle’s path change? (Assume fields in the velocity selection region are unchanged.) It will: (show your work) Halve - stay the same – double – quadruple - other ...
... (f) If the magnetic field B in the deflection region doubles, how will the RADIUS of curvature of the particle’s path change? (Assume fields in the velocity selection region are unchanged.) It will: (show your work) Halve - stay the same – double – quadruple - other ...
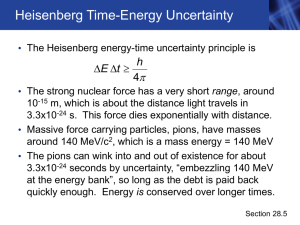
Uncertainty not so certain after all Early formulation
... Review Letters. “It’s really just this [one aspect] that needs to be updated.” In its most famous articulation, Heisenberg’s uncertainty principle states that it’s possible at a given moment to know either the position or momentum of a particle, but not both. This relationship can be written out mat ...
... Review Letters. “It’s really just this [one aspect] that needs to be updated.” In its most famous articulation, Heisenberg’s uncertainty principle states that it’s possible at a given moment to know either the position or momentum of a particle, but not both. This relationship can be written out mat ...