* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Energy Unit PowerPoint
Open energy system models wikipedia , lookup
William Flynn Martin wikipedia , lookup
Energy storage wikipedia , lookup
100% renewable energy wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Energy subsidies wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Potential energy wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Regenerative brake wikipedia , lookup
Low-carbon economy wikipedia , lookup
Kinetic energy wikipedia , lookup
World energy consumption wikipedia , lookup
Energy policy of Australia wikipedia , lookup
Energy harvesting wikipedia , lookup
Alternative energy wikipedia , lookup
Energy policy of Finland wikipedia , lookup
International Energy Agency wikipedia , lookup
Distributed generation wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Internal energy wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Negawatt power wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Conservation of energy wikipedia , lookup
Energy efficiency in British housing wikipedia , lookup
United States energy law wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
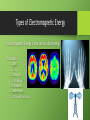
th 7 Grade Energy Unit Chapter Five, Section One What is Energy? What is Work? • Work is done on an object when the object moves in the same direction in which the force is exerted. Requirements for work to occur: 1. 2. Object that the force is being applied to has to move Force is in the same direction as the object moving. Object is moving in the same direction as the force. Energy, Work, and Power • The ability to do work or cause a change is called energy. What are some examples of work that you have done today? Work and Energy • When one thing does work on another some of its energy is transferred to that object. Work and Energy • In other words, work is the transfer of energy. • The object which the work is done upon gains energy. • Energy is measured in JOULES (J) • http://www.brainpop.com/science/motionsforcesandtime/work/p review.weml What is the origin of all energy? •THE ORIGIN OF ALL ENERGY IS THE SUN AND THE EARTH’S CORE Kinetic Energy • Two basic kinds of energy are: 1. Kinetic energy 2. Potential energy • To determine if energy is potential or kinetic, you must first determine if the object is in motion. Kinetic Energy Kinetic Energy • Because the moving object does work, it has energy. • The energy that an object has due to its motion is called kinetic energy • The word kinetic comes from the Greek word kinetos, which means moving. • http://www.brainpop.com/science/energy/kineticenergy/preview .weml Factors Affecting Kinetic Energy • There are two main factors that affect the amount of kinetic energy that an object has: 1. Mass 2. Velocity Which rock would have the greater amount of kinetic energy when rolled down a hill? Factors Affecting Kinetic Energy Factor How the Kinetic Energy is Affected Increase in Mass Increases the amount of Kinetic Energy Decrease in Mass Decreases the amount of Kinetic Energy Increase in Velocity Increases the amount of Kinetic Energy Decrease in Velocity Decreases the amount of Kinetic Energy A Good Hint • If you do not know the mass and velocity of an object and want to know if it has a lot of a little kinetic velocity, ask yourself the following question: “How hard would it be to stop the object from moving?” • The harder it is to stop an object from moving the more kinetic energy it is going to have. Calculating Kinetic Energy Kinetic Energy = ½ × Mass × Velocity² • The formula above means that Mass and Velocity do not have the same effect on the kinetic energy of an object. • The velocity will have a greater impact than the mass of the object. Potential Energy • The second basic type of energy is Potential Energy • Stored energy that results from the position or shape of an object is called potential energy. • This type of energy has the potential to do work. Potential Energy • There are two main divisions of potential energy: • Gravitational Potential Energy • Elastic Potential Energy http://www.brainpop.com/science/energy/potentialenergy/preview.weml Forms of Energy Chapter Five, Section Two Types of Potential Energy Potential Energy is stored Energy 1. 2. 3. 4. Gravitational Potential Energy Elastic Potential Energy Chemical Energy Nuclear Energy Gravitational Potential Energy • Potential Energy that is related to an object’s height is called gravitational potential energy. • Gravitational potential energy = do the work needed to lift it Gravitational Potential Energy = Weight × Height Gravitational Potential Energy • Examples of Gravitational Potential Energy: 1. Rollercoaster at the top of the hill 2. Water being held back by a dam 3. Rock on the edge of a cliff Question • Which has greater gravitational potential energy ---a book lifted 1 meter off the ground or the same book lifted 10 meters off the ground? • Can you prove it? Calculations • Wrecking ball (A) has a weight of 700N and hangs at a height of 100 meters. • Wrecking ball (B) has a weight of 800N and hangs at a height of 80 meters. Which has more gravitational potential energy? Elastic Potential Energy • An object gains a different type of potential energy when stretched. • The potential energy associated with objects that can be stretched or compressed is called elastic potential energy • Also known as stored mechanical energy. Elastic Potential Energy • Examples of Elastic Potential Energy: 1. Rubber bands 2. Springs 3. English long bow Chemical Energy • Chemical compounds are made up of atoms and molecules • Bonds hold atoms and molecules together. • THESE BONDS HAVE STORED CHEMICAL ENERGY. • https://www.youtube.com/watch?v= y_omJiYB2gk Chemical Energy • Chemical Energy is potential energy stored in chemical bonds that hold chemical compounds together. • Examples of chemical Energy 1. Food 2. Fossil Fuels 3. Batteries Nuclear Energy • Nuclear Energy – is a type of potential energy that is stored in the nucleus of an atom. • Nuclear Energy is released during a nuclear reaction. • https://www.youtube.com/watch?v=zsTRxXvQY0s Nuclear Energy • Examples of Nuclear Energy: 1. The sun 2. Atomic bomb 3. Nuclear Power plants Types of Kinetic Energy • Kinetic Energy is the energy of motion. 1. 2. 3. 4. Electrical Energy Electromagnetic Energy Thermal Energy Mechanical Energy Electrical Energy • The energy of electric charges is electrical energy • Electrical Energy is caused by the movement of electrons. Electrical Energy • Examples of Electrical Energy: 1. Lightning 2. Electric current 3. Appliances that run on electricity Electromagnetic Energy • Electromagnetic Energy is energy that comes in form of the electromagnetic spectrum. • The electromagnetic spectrum is the range of all possible Electromagnetic radiation. Types of Electromagnetic Energy • Electromagnetic Energy is also called radiant energy. • Examples: 1. 2. 3. 4. 5. 6. 7. Light X-rays Radio rays Infrared rays Microwaves Gamma rays Ultraviolet radiation Thermal Energy • All objects are made up of atoms and molecules and because these particles are constantly in motion, they have kinetic energy. Which box below has more kinetic energy? Why? Thermal Energy • The faster they more the more kinetic energy an object has. • The total potential and kinetic energy of the particles of an object are called thermal energy. Thermal Energy Examples: Lava – Lava moving down the side of the hill may be moving slowly but its particles that make up the lava are moving quickly. Because the particles have a large amount of kinetic energy the lava has a large amount of thermal energy. Mechanical Energy • The form of energy that is a associated with the position and motion of an object is called mechanical energy. • An objects mechanical energy is a combination of its potential energy and kinetic energy. Mechanical Energy = Potential Energy + Kinetic Energy Thermal Energy and Heat Chapter Six, Section One Thermal Energy and Heat Different objects at the same temperatures CAN have different energies. Thermal Energy • Remember that the total energy of all particles in an object is the thermal energy. • Thermal energy depends on: 1. The number of particles in the object 2. The arrangement of the object’s particles. Thermal Energy • The more particles an object has at a given temperature, the more thermal energy it has. The containers to the right are identical temperature, which the least thermal energy? Why? Heat • Thermal energy that is transferred from matter at a higher temperature to a lower temperature is called heat • Only when thermal energy is transferred is it called heat • Heat is thermal energy moving from a warmer object to a cooler object. Heat Energy Transformation and Conservation Chapter Five, Section Three Energy Transformation • Most forms of energy can be transformed into other forms. • A change from one form of energy to another form of energy is called an energy transformation. • Types of energy transformation: 1. Single transformations 2. Multiple transformations Single Transformations • Sometimes one form of energy needs to be transformed into another to get work done. • Remember: work is force exerted on an object causing it to move • Examples: 1. Turbines moving in the dam turning mechanical energy into electrical energy 2. Your body converts the chemical energy in food into thermal energy that your body uses to maintain its temperature. What is the single transformation? Multiple Transformation • Often a series of energy transformations are needed to do work(make something move). • Examples: 1. The energy needed to strike a match is transformed first to thermal energy. The thermal energy causes particles in the match to release the stored chemical energy, which is transferred into thermal energy and the electromagnetic energy you see as light Energy Chains • Energy cannot be created or destroyed it is never transformed just once. • Energy is transformed from one type to another to another and so on. • When we follow these transformations we get an energy chain. Energy Chain Transformation Between Potential and Kinetic Energy • The most common energy transformations is the transformation between potential and kinetic energy. Transformation Between Potential and Kinetic Energy Example Energy Transformation in Juggling: • Any object that rises or falls experiences a change in gravitational potential energy and kinetic energy. 1. As the ball rises it slows down-kinetic energy decreases. Since the ball is going higher in the air, the potential energy increases. 2. At the highest point the ball stop moving for a split second, at that point there is no kinetic energy, and its potential energy is at the highest point. 3. As the ball falls there is a loss in altitude, so the potential energy decreases, at the same time the ball is moving faster and faster, so the kinetic energy increases. Energy Transformation in Juggling Energy Transformation in a Pendulum • Can you explain how kinetic and potential energy change in the picture to the left? Energy Transformation in a Pole Vault • Can you explain how kinetic and potential energy change in the picture to the left? Conservation of Energy • The Law of Conservation of Energy states that when one form of energy is transformed into another, no energy is destroyed in the process. • According to the law of conservation of energy, energy cannot be created nor can it be destroyed. https://www.youtube.com/watch?v=LrRdKmjhOgw Conservation of Energy • This means: 1. Energy is the same before and after the transformation 2. If you add up all the new forms of energy = the old forms of energy Energy and Friction • Whenever a moving object experiences friction, some the kinetic energy is transformed into thermal energy. Energy and Fossil Fuels Chapter Five, Section Four Formation of Fossil Fuels • The plants of vast forests that at one time covered Earth provide the energy stored in fuels. • A fuel is a material that contains stored potential energy. • Examples: 1. Gas 2. Lumber Formation of Fossil Fuels • Some fuels used today were made hundreds of millions of years ago. • These fuels, which include coal, petroleum, and natural gas are known as fossil fuels. Formation of Fossil Fuels • Fossil Fuels are made of hydrocarbons • Hydrocarbons – are chemical compounds that contain hydrogen and carbon atoms. • The combustion of fossil fuels provides more energy per kilogram than does the combustion of other fuels. Relative Amounts of Energy • Coal • twice the energy of wood • Oil and natural gas • three times the energy of wood Coal • Coal is a solid fuel formed from plant remains. Coal • Coal is the most plentiful fossil fuel in the United States. • Advantages • Easy to transport • Provides a lot of energy • Disadvantages • Causes erosion • Runoff causing water pollution • Air pollution Oil • Oil is a thick, black, liquid fossil fuel. Oil • Oil forms from the remains of small animals, algae, and other organisms that lived in oceans and shallow inland seas hundreds of million years ago. • Petroleum is another name for oil Oil • Petroleum accounts for more then 1/3 of energy produced in the world. • What uses petroleum: • • • • • Cars Homes Boats Airplanes Trains Where is Oil Located? • Most oil deposits are located underground in tiny holes in sandstone or limestone. Where is Oil Located? • Located deep below the surface • Finding oil is difficult • Use sound waves to find oil • One of every six wells drilled produces oil of useable amount Origins of U.S. Oil Natural Gas • Natural Gas is a mixture of methane and other gases. • Formed from the same organisms as oil • Less dense than oil so it is normally found directly above it in deposits Natural Gas Natural Gas • Can be compressed and made into a liquid to put in tanks for fuel. • Advantages: • Large amount of energy • Low pollution • Easy to transport • Disadvantages • Highly flammable Fuel Supply and Demand • Oil is essential to modern day life • Since fossil fuels take hundreds of millions of years to form they are considered nonrenewable resources. • The oil we have today took about 500 million years to form. • ¼ of the oil we have is already gone • Eventually all the oil on earth will be used up. Fuel Supply and Demand • Many nations consume huge amounts of fossil fuels and have a very small reserve. • United States: • Uses 1/3 of all oil used in the world • Only has 3% of the world’s oil supply