* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharmacokinetics
Survey
Document related concepts
Discovery and development of proton pump inhibitors wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Compounding wikipedia , lookup
Orphan drug wikipedia , lookup
Plateau principle wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Transcript
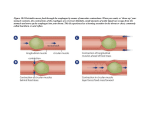
CLASS: 11-12 DATE: Novermber 29, 2010 PROFESSOR: Prasain General Principal 1: Pharmacokinetics Scribe: Kratika Pareek Proof: Angi Gullard Page 1 of 3 I. GENERAL PRINCIPAL 1: PHARMACOKINETICS [S1] a. Let’s start with some of the basic things: what is the relevance of studying pharmacokinetics? b. So if you have a headache or any other disease than what is the first thing that we need to know in order to treat this disease? First thing we need to know is what medicine is best for what we have. c. We also need to know how many pills we need to take it depends on your condition. d. We also need to know how often to take the medicine for and how long to take it for. e. So there are 3 How’s: how much, how often, and how long, that we need to consider in order to have the optimal effect of the drug. f. Too low will have no effect and too much can have a toxic effect. II. OUTLINES [S2] a. Talk about basic understanding of pharmacokinetics (PK) b. Read slide. III. WHAT IS PHARMACOKINETICS (PK)? [S3] a. PK is our body’s action on the drug, how our bodies respond to the drug, and dynamics of drug from the site of administration of drug to the site of action as well as its elimination. b. (Drawing on board): concentration on y and time on X axis. Lets say you take 2 pills of Motrin and over time you want to see the level of that drug in the blood. So over time it reaches its peak level and slowly its concentration decreases. There is a time where you have maximum concentration. PK is the function of concentration and time. c. There are several steps when you take a drug: absorption, distribution, metabolism and elimination. The other aspects are effects and doses. d. Today we will talk about drug concentration and time. And when there is kinetics, there is a time factor. e. 4 important things for PK behavior of drug: i. Absorption = its first and foremost important to execute any biological effect. Absorption means entry of drug from site of administration to site of tissue or action or receptor. Absorption is very important because the way you take any drug (oral, IV) dictates how much gets into the circulation. ii. Distribution = means from blood stream to tissue. This dictates how you clearly can remove the drug. iii. Metabolism = dictates the amount taken. Some drugs you have to take a lot and some only small amounts. What is the need for metabolism? mostly drugs are small molecule lipophilic drugs i.e. water insoluble. It’s easy to take drugs inside but its difficult to remove them. Drugs have Xenobytes for extended periods of time and you don’t want any of this, so once the effect of the drug is done the drug should be removed. The central point is that mostly drugs are lipophilic. Metabolism comes down to how you eliminate drugs and for that the drugs should be water-soluble so that they can dissolve in water and come out. iv. Elimination = once the effect is done the chemicals need to be removed from the body or they can have other undesirable/toxic effects. Elimination is important to decide what dose to take in. IV. HOW ARE DRUGS ADMINISTERED? [S4] a. The real question is how we take drugs in the first place: oral or IV. b. First thing we prefer is oral. However, there are still choices that can be preferred over some. c. These choices have advantages and disadvantages. d. There is no single administration that is good for all the drugs. e. Administration is dictated by physiochemical activity of the drug. V. ENTERAL ROUTE (MOST CONVENIENT, ECONOMIC AND SAFE) [S5] a. Enteral is a Latin word related to GI tract. When we say enteral, drugs go through GI tract. b. In broad sense, it includes oral and sublingual (under the tongue) and rectal. Sublingual is still enteral because enteral is when there is administration within mouth and rectum. c. Oral is the most preferred way because it’s easy to take it and easy in the sense that you don’t have to think about putting IV that requires help from professionals. It’s also convenient and safe and economic. d. The problem with oral is that when you take drugs orally, it’s almost impossible to take 100% of it into the circulation (when you inject IV, it goes directly into circulation). e. One good thing about oral compared to IV is that when a toxic thing is taken orally it can still be changed before it enters circulation. f. One of the disadvantages is bioavailability, which is associated with first pass metabolism. g. First pass metabolism = nature has designed our bodies in such a way that it tries to remove xenobytes. So oral is a natural way. Oral goes through GI route and enters circulation through the liver. There is an organ CLASS: 11-12 Scribe: Kratika Pareek DATE: Novermber 29, 2010 Proof: Angi Gullard PROFESSOR: Prasain General Principal 1: Pharmacokinetics Page 2 of 3 that is involved here. First it must go to liver. Most of the drugs undergo metabolism and liver derivitizes the drug into different metabolites. So the net concentration you see in the blood is always less than what you took orally. So the bioavailability of the drug is less than 100% and is thus a major issue. h. This means that elimination has already started in this first pass. This is the pre systemic elimination. There is a possibility that once taken orally the metabolism in the first pass mechanism is a major issue. i. Other thing with orally is onset of the effect is slow and if not preferred under emergency. And also there is decomposition of peptides by the stomach enzymes. So this is another issue. j. Sublingual is not very common. Ex, nitroglycerine. Sublingual is also enteral, but It avoids the first pass. Its onset is quick and directly goes to blood circulation and so you can inject very small volume. k. Rectal is used in pediatrics with special children who have problems with swallowing. l. Now lets talk about outside GI: the paraenteral route, which includes subcutaneous, intra muscular and intra venous. It’s important in the case of emergency and when a person is unconscious. There is no first pass effect here. Limitation is that what you inject you can’t take back. Also the contamination of the IV is a major issue. There is also an issue with toxicity so you need a professional to administer it and it’s expensive. m. Other routes of absorption are topical, inhalation. Inhalation is very effective and it goes directly into lungs. Also transdermal, nicotine pads, hormone replacement therapy. VI. ABSORPTION [S6] a. Drug absorption is the movement of the drug from its site of administration to bloodstream. b. Drug absorption is decided by physical and chemical properties of a drug. i. Most drugs are small molecules with a MW of less than 1000 Da. ii. Most are weak acids and weak bases and thus are not soluble in water. iii. They have to cross the phospholipid bilayer. The membrane has lipids and so lipophilic drugs can penetrate and be absorbed faster and in larger quantity. c. The movement of drug from GI to blood is passive diffusion going form high concentration gradient to low. When you have small lipophilic drugs that can easily go from high concentration in the GI to low concentration in the blood. i. This concentration gradient is the driving force. ii. Absorption for most of the drugs takes place by this. iii. There is not saturation because there is no involvement of any proteins. d. In order to get absorbed the drug should NOT be in ionized form. Ex, aspirin. It’s a weak acid and will have no absorption in another acid. So the unionized form is easy to absorb and passive diffusion would not be possible in ionized molecules. e. There are carrier proteins that take drugs to circulation and this is called active transport. It involves several transporters both sodium dependent and sodium independent. i. It takes a compound against its concentration gradient. ii. It’s saturable meaning there are limited number of proteins and when those proteins are occupied carrying the drug it reaches its saturation point (unlike passive diffusion) f. Take home message: passive absorption is a major way that most of the drug metabolites and small insoluble lipophilic drugs and unionized molecules get into the body by passive diffusion. Some compounds are transported by transport proteins and is saturable mechanism. VII. FACTORS AFFECTING ORAL ABSORPTION OF A DRUG [S7] a. When you take a solid drug orally or in suspension which one will get absorbed faster. Absorption for a solid drug is not possible until it gets in soluble form. So the first even is disintegration or dissolution. b. Disintegration means how quickly a drug gets into soluble form. c. Other thing is pH. It alters the ionization form. When pH increases, ionization happens. d. Surface area is also an important factor. Small intestine has a higher surface area. So aspirin is absorbed more in the small intestine even though pH is lower in the stomach. e. Gastric emptying = there is a transit between stomach and intestine. Gastric emptying if different if you take a drug with food and another drug by itself. If the drug remains in the stomach for a longer period of time then absorption in the small intestine is delayed. f. So these are the factors that affect how quickly and how often a drug is observed. VIII. WHAT ARE pH AND pK? [S8] a. pK tells the ability of a molecule to undergo acid base chemistry, if they can ionize and how sensitive they are. i. Low pK = acid ii. High pK = base. CLASS: 11-12 DATE: Novermber 29, 2010 PROFESSOR: Prasain b. c. d. e. f. General Principal 1: Pharmacokinetics Scribe: Kratika Pareek Proof: Angi Gullard Page 3 of 3 pH is the hydrogen ion concentration. Unprotonated means unionized and protonated means ionized form. pH = pKa + log [unprotonated form] / [ protonated form] pKa and pH would be same when there is 50% ionized and 50% unionized form. So its very important to understand the pH and pK values of drug to understand how its absorbed in the small intestine. IX. ROLE OF pH IN DRUG ABSORPTION [S9] a. HA is any weak acid. So what happens in the stomach there is very low pH here. Its mostly unionized form. b. Plasma is neutral or a little basic. So when it reaches the plasma there is more ionized form present because the pH is higher. c. Bases always take proton so when there is a base then more protons will be taken away and there will be more ionized form present. d. Acids ionize none in stomach, a little in intestine and more in blood. Because of this absorption is possible. e. With bases, there is more ionization in stomach and less in the intestine and almost none in blood. X. ABSORPTION OF ASPIRIN FROM STOMACH [S10] a. pKa of aspirin is 3.4 and pH is -2 (derived from the equation). Log of -2 is .01. This means that HA is 100 fold greater than A- ion. So in the stomach the unionized form is 100 fold higher than ionized form. And thus it moves from stomach to blood. b. Once you know the pKa value, and you know the pH of the stomach and the intestine, you can predict the fate of absorption in stomach and intestine. c. So we can find out what drugs have high pKa and what is the ratio in the stomach and the small intestines. d. Question from student: why would it move from the stomach to the blood? Is it just a concentration difference? Answer: The thing is that it is unionized. If it was ionized then it would be impossible. So there is a possibility that it can move from the stomach to blood. It can’t move with passive diffusion because it’s ionized. So it is not the concentration. It has to do with it being not ionized. We are talking about the role of the pH here that controls some absorption. [End 52:25 mins]