* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch16
Basal metabolic rate wikipedia , lookup
Biosequestration wikipedia , lookup
Butyric acid wikipedia , lookup
Biosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Photosynthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
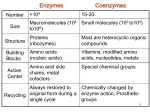
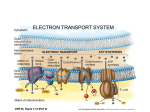
Selected Solutions to End of Chapter 16 Problems Class 1 1. Here is the answer, but be sure you know these chemical structures of substrates and products, but not necessarily the enzyme cofactors. Citrate Synthetase: a. Acetyl-CoA + Oxaloacetate + H2O Citrate + CoA b. CoA. c. condensation. Aconitase: a. Citrate Isocitrate b. none. c. isomerization Isocitrate Dehydrogenase: a. Isocitrate + NAD+ α-ketoglutarate + CO2 + NADH b. NAD+ c. oxidative decarboxylation. α-Ketoglutarate Dehydrogenase: a. α-ketoglutarate + NAD+ + CoA succinyl-CoA + CO2 + NADH b. NAD+, CoA, thiamin pyrophosphate. c. oxidative decarboxylation Succinyl-CoA synthetase: a. succinyl-CoA + Pi + GDP succinate + CoA + GTP b. CoA. c. substrate level phosphorylation Succinate Dehydrogenase: a. Succinate + FAD Fumarate + FADH2 b. FAD. c. oxidation. Fumarase: a. Fumarate + H2O malate b. None. c. hydration. Malate Dehydrogenase: a. Malate + NAD+ Oxaloacetate + NADH b. NAD+ c. oxidation Net: Acetyl-CoA + 3 NAD+ + FAD + GDP + Pi + 2 H2O 2 CO2 + CoA + 3NADH + FADH2 + GTP 2. Now lets get this major part of Central Metabolism all Summed Up: Glycolysis: Glucose + 2 ADP + 2 Pi + 2 NAD+ 2 pyruvate + 2 ATP + 2 NADH Pyruvate Dehydrogenase: 2 Pyruvate + 2 NAD+ + 2 CoA 2 acetyl-CoA + 2 CO2 + 2 NADH Citric Acid Cycle: 2 Acetyl-CoA + 6 NAD+ + 2 FAD + 2 Pi 2 CoA + 4 CO2 + 6 NADH + 2 FADH2 + 2 GTP OVERALL: (converting GTPs to ATPs) Glucose + 4 ADP + 4 Pi + 10 NAD+ + 2 FAD 6 CO2 + 4 ATP + 10 NADH + 2 FADH2 3. Oxidation or Reduction? a. oxidation (alcohol to carbonyl). b. oxidation (carbonyl to carboxyl) c. reduction (CO2 to carboxyl) d. reduction (carboxyl to carbonyl) e. oxidation (alcohol to carbonyl) f. oxidation (methyl to carboxyl) g. oxidation (ane to ene) h. oxidation (carboxyl to CO2) 4. Compare metabolism of glucose (C6H12O6) and hexanoic acid (C6H14O2). This is a sugar compared to a short chain fatty acid (we will see this β-oxidation pathway in Chapter 17). Answer: Consider which one is the most reduced, it has more electrons to give though oxidation reactions (energy producing). It should be obvious that it is hexanoic acid. Later we will see that the amount of energy produced from fatty acids (β-oxidation and respiratory electron transport) is far more than through glycolysis + pyruvate dehydrogenase + citric acid cycle + respiratory electron transport)…all because of the redox state of fatty acids (mostly methyls) vs sugars (mostly alcohols). 5. Use of NADH and NAD+ in some of the reactions in Problem 3 or others. (Note when writing NADH you realize it is really NADH + H+), this way it makes it shorter to just write NADH.) a. oxidized: ethanol + NAD+ acetaldehyde + NADH b. reduced: 1,3 bisphosphoglycerate + NADH glyceraldehyde-3-phosphate + NAD+ + Pi c. unchanged: pyruvate acetyl-CoA + CO2 (NAD+ not used in this reaction, all electron on products). d. oxidized: puryvate + NAD+ acetate + CO2 + NADH e. reduced: oxaloacetate + NADH malate + NAD+ f. unchanged: acetoacetate + H+ acetone + CO2 6. Describe the co-factor reactions in pyruvate dehydrogenase (enzymes 1, 2 and 3): TPP – thiamine pyrophposhpate ring adds to and stabilizes the carbanion. Lipoic acid – oxidizes pyruvate to acetate and activates CoA as a thioester. CoA-SH - becomes the thioester. FAD – oxidizes reduced lipoic acid. NAD+ - oxidizes FADH2 to FAD becoming NADH +H+. 7. Thiamine deficiency patients (part of the classroom work) have high levels of pyruvate in blood. Why? It’s because some of the pyruvate can not be converted to acetyl-CoA and is exported from cells to blood. Note that this also makes α-ketoglutarate dehydrogenase much less active because it has the same mechanism as pyruvate dehydrogenase. 8. Isocitrate dehydrogenase is an oxidative decarboxylase using either NAD+ or NADP+ as the electron acceptor. But its mechanism does not use TPP, lipoate, FAD, CoA, etc. like pyruvate DH or α-ketoglutarate DH. 9. This problem integrates the other function of the Citric Acid Cycle (CAC): CAC intermediates are used as the starting molecules for several amino acid biosynthesis and pyrole (heme) biosynthesis. This drains CAC intermediates away which could slow down CAC (CAC can only move if there is sufficient oxaloacetate OAA). So when OAA or malate were added to pigeon muscle preps, their oxygen uptake increased because the CAC got moving faster (having α-ketoglutarate, succinyl-CoA, and OAA syphoned off for biosynthesis, reduces the amount of OAA to be the acceptor for acetylCoA). We will cover here the anaplerotic reactions that replace the CAC intermediates so the OAA concentration is increased and stabilized. Because CAC is in the mitrochondrion, most all of the NADH and FADH2 produced by the CAC is immediately used by the electron transport system (cytochromes) to generate the proton motive force using the energy of the electrons in NADH and FADH2 as they electron move through to eventually reduce oxygen to water. Thus mitochondrial oxygen uptake is a measure of electron transport system supplied by CAC and a bit by Glycolysis. 15. Malonate was one, if not the first, enzyme inhibitor whose kinetics were studied. Compare malonate to succinate: _OOC-CH -COO2 to -OOC-CH -CH -COO2 2 What type of inhibition would you suspect? a. competitive. b. uncompetitive. or c. mixed? 18. Another follow the carbon labeling experiment through Glycolysis and CAC. Where is 1-14Cglucose in: a. Fructose-1,6-bisphosphate? Carbon 1 b. Glyceraldehyde-3-phosphate? Carbon 3 c. Phosphoenolpyruvate? Carbon 3 d. Acetyl-CoA? Carbon 2 (the methyl carbon) e. Citrate? Carbon 2 f. α-ketoglutyrate? Carbon 4 g. Oxaloacetate? Carbon 2 and 3 (half and half distribution). 19. We have touched on this already: thiamine deficiency…the disease Beri Beri, and will do more in class. Thiamine is obviously needed for the synthesis of thiamine pyrophosphate used in the two dehydrogenases for pyruvate and α-ketoglutarate. 30. Coupling of CAC and electron transport (respiration). When oxygen is low or if electron transport is inhibited, the CAC slows or stops. Why? This is easy to think about, without electron transport taking electrons from NADH and FADH2 the concentration of these cofactors increases with the decrease of NAD+ and FAD (without which the CAC cannot function). High [NADH] inhibits reactions leading into and in the first part of the CAC….flow into the next question. 31. Additionally, the reduced forms are inhibitors of the control points in CAC: pyruvate dehydrogenase, citrate synthase, and α-ketoglutartate dehydrogenase. 32. Lets get to the actual ΔG of citrate synthase in the mitochondria where [acetyl-CoA] = 1µM, [OAA] = 1 µM, [citrate] is 220 µM, and [CoA] = 65 µM. The ΔG o’ = -32.2 kJ/mole which indicates it should go forward fairly well. Remember ΔG = ΔGo’ + RT lnQ Q= [citrate][CoA] [OAA][acetyl−CoA] Q = 1.48 x104 = (220 x10−6 )(65 x 10−6 ) (1 x 10−6 )(1 x10 −6 ) so lnQ = 9.60 ΔG = -32.2 kJ/mole + 2.48 kJ/mole (9.6) = -32.2 kJ/mole + 23.8 kJ/mole ΔG = -8.4 kJ/mole therefore still exothermic (=exergonic).