* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Solid Tumour Section Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma
Epigenomics wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Gene expression profiling wikipedia , lookup
Protein moonlighting wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Genetic engineering wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Primary transcript wikipedia , lookup
Gene therapy wikipedia , lookup
Genome (book) wikipedia , lookup
Non-coding DNA wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
DNA vaccination wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Oncogenomics wikipedia , lookup
Microevolution wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Point mutation wikipedia , lookup
Designer baby wikipedia , lookup
Helitron (biology) wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
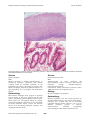
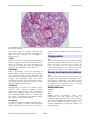
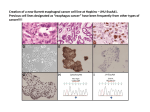
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Solid Tumour Section Mini Review Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma DunFa Peng, Wael El-Rifai Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA (DP, WER) Published in Atlas Database: August 2009 Online updated version : http://AtlasGeneticsOncology.org/Tumors/BarrettsEsophagID5591.html DOI: 10.4267/2042/44810 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2010 Atlas of Genetics and Cytogenetics in Oncology and Haematology Etiology Identity Gastroesophageal reflux disease (GERD) is considered the major risk factor for Barrett's esophagus. About 1 in 10 patients with GERD are found to have Barrett's esophagus. GERD generates reactive oxygen species that produce oxidative stress and subsequent oxidative DNA damage. Some DNA damage may cause DNA mutations that accumulate and cause tumor formation. Note: Barrett's adenocarcinoma is one of malignancies with the most rapid increase in incidence during past decades in the Western countries. It is defined as adenocarcinoma of the lower esophagus and gastroesophageal junction associated with Barrett's esophagus. Barrett's esophagus is the only known precursor for Barrett's adenocarcinoma through Barrett's dysplasia (also called metaplasia-dysplasiaadenocarcinoma sequence). Epidemiology Barrett's esophagus is more common in men than in women, with a male:female ratio of about 2:1. The risk factors of Barrett's esophagus include Age (increasing with age), Race (more common in Caucasians), smoking (not clear), alcohol consumption (not clear), gastroesophageal reflux disease (GERD, major factor), and obesity. On the other hand, some controversial reports indicate that H. pylori infection and the virulent cagA strains in particular, may protect against the development of Barrett's oesophagus and progression to adenocarcinoma. Clinics and pathology Disease Barrett's Esophagus Note Barrett's esophagus is defined as the normal esophageal squamous epithelium that is replaced by intestinalized metaplastic columnar epithelia. Phenotype / cell stem origin Clinics The progenitor cell from which Barrett's oesophagus develops is still unclear. Progenitor cells resident in the submucosal glands or the interbasal layer of the epithelium, bone-marrow-derived stem cells, or transdifferentiated squamous cells are included in the candidate cells. Atlas Genet Cytogenet Oncol Haematol. 2010; 14(7) Heartburn is the most common symptom of GERD and Barrett's esophagus. 698 Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma Peng D, El-Rifai W Normal esophagus is covered by squamous epithelia (A). However, in Barrett's esophagus (B), the squamous epithelia are replaced by intestinalized metaplastic columnar epithelia. Disease Disease Barrett's dysplasia Note Barrett's dysplasia is defined morphologically as unequivocal neoplastic epithelium that remains confined within the basement membrane of the epithelium from which it developed. In patients with Barrett's esophagus, dysplasia is graded as either low or high, depending on its cytological and architectural features. Barrett's adenocarcinoma Note Adenocarcinoma of lower esophagus and gastroesophageal junction associated with Barrett's esophagus through metaplasia-dysplasiaadenocarcinoma sequence. Classification: Tumor classification is based on UICC TNM classification for esophageal cancers. Etiology Epidemiology Barrett's esophagus with dysplasia Most Barrett's esophagus never progress to dysplasia and carcinoma. It has been reported that the male gender, longstanding gastroesophageal reflux disease, hiatal hernia size, and segment length are strongly associated with Barrett's dysplasia. On the other hand, successful antireflux surgery protects the Barrett's mucosa from developing high-grade dysplasia and esophageal adenocarcinoma. Atlas Genet Cytogenet Oncol Haematol. 2010; 14(7) Epidemiology Barrett's esophagus is the only known precursor for Barrett's adenocarcinoma. The patients with Barrett's esophagus have 20 folds more risk for developing esophageal adenocarcinoma. However, only 1-5% of Barrett's esophagus progress to Barrett's adenocarcinoma. Although longstanding gastroesophageal reflux disease, hiatal hernia size, 699 Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma Peng D, El-Rifai W A representative image of a Barrett's adenocarcinoma with moderate to poor differentiation. Atypic tumor cells form quite irregular tubules and some form solid cord. and segment length are strongly associated with adenocarcinoma, Barrett's esophagus with dysplasia is likely the true precursor for developing to adenocarcinoma. patients amenable to definitive treatment ranges from 5 to 30%. Cytogenetics Clinics Note The chromosomal alterations most frequently identified in Barrett's adenocarcinoma by CGH were: gains in 8q (80%), 20q (60%); 2p, 7p and 10q (47% each), 6p (37%), 15q (33%), and 17q (30%). Losses were observed predominantly in the 4q (50%); 5q and 9p (43% each), 18q (40%), 7q (33%), and 14q (30%). Heartburn is the most common symptom of GERD and Barrett's esophagus. As for Barrett's adenocarcinoma, it shares the symptoms with other esophageal type cancers, such as difficulty swallowing, unexplained weight loss, pain in the throat or mid-chest, etc. Pathology Genes involved and proteins There is no difference in the term of histology of Barrett's adenocarcinoma from that in the stomach and colon. It can be graded into well-, moderately- and poorly-differentiated adenocarcinoma based on their cytologic and architectural atypia. The Lauren classification for gastric cancer has also been used by some pathologists and physicians to divide into either intestinal or diffuse histological type. Note There are many genes that have been reported to be genetically and/or epigenetically dysregulated, such as gene mutation, amplification, and LOH and DNA methylation that involved cell cycle control, apoptosis, cell adhesion and antioxidative stress, etc. Some representative genes are described below. Treatment ERBB2 (HER2/neu) Esophagectomy is still the most common primary treatment. Other treatment modalities include chemotherapy, radiation therapy, stents, photodynamic therapy, and endoscopic therapy with an Nd:YAG laser. Combined modality therapy (i.e., chemotherapy plus surgery or chemotherapy and radiation therapy plus surgery) is under clinical evaluation. Location 17q21 Note ERBB2 protein over-expression and/or DNA amplification have been reported in 10-70% of esophageal adenocarcinomas. The present literature data suggest that ERBB2 overexpression may be a late event in the dysplasia-carcinoma sequence, as it occurred predominantly in high-grade dysplasia and adenocarcinomas. Prognosis The prognosis of Barrett's adenocarcinoma depends on the stage at diagnosis, treatment and the patients' general condition. The overall 5-year survival rate in Atlas Genet Cytogenet Oncol Haematol. 2010; 14(7) 700 Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma Peng D, El-Rifai W CGH analysis of a case of Barrett's esophageal adenocarcinoma. Tumor DNA was labeled with FITC (Green) and reference DNA was labeled with TRITC (red). The hybridizations were analyzed using an Olympus fluorescence microscope and the ISIS digital image analysis system (Metasystems GmbH, Altlussheim, Germany) based on integrated high-sensitivity monochrome CCD camera and automated CGH analysis software. The green colon indicates areas of DNA gains whereas the red color indicates DNA losses in the tumor sample. It has been reported that ERBB2 overexpression/amplification in carcinoma correlated significantly with tumor invasion, lymph node metastasis, and poor prognosis in patients with Barrett's related adenocarcinoma. Protein The ERBB2 (also called HER2 or NEU) gene encodes an integral type I protein of 185 kDa, 1255 amino acids, with a cysteine-rich extracellular ligand-binding domain, a transmembrane domain and an intracellular region endowed with a tyrosine kinase activity. domain, a nuclear localization signal, a basic domain, a helix-loop-helix motif, and a leucin zipper. CDX1 Location 5q33.1 Note CDX1 is predominantly expressed in the small intestine and colon, but not in normal esophageal squamous epithelia or gastric epithelia. CDX1 was over-expressed in Barrett's esophagus and adenocarcinomas, most likely through promoter DNA hypomethylation. Protein Member of the caudal-related homeobox transcription factor gene family. The encoded protein regulates intestine-specific gene expression and differentiation of intestine. C-MYC Location 8q24 Note Frequent high-level chromosomal amplification of 8q21 has been reported in Barrett's adenocarcinoma and c-myc is the potential target gene for this amplification. It has been reported that amplification of c-myc was detected in 25% of high-grade dysplasia and 44% of adenocarcinomas, but in none of Barrett's metaplasia and low-grade dysplasia. Protein DNA binding protein with 439 amino acids and 48 kDa (p64); 454 amino acids (p67, 15 additional amino acids in N-term), contains a transactivation domain, an acidic Atlas Genet Cytogenet Oncol Haematol. 2010; 14(7) CDX2 Location 13q12.2 Note CDX2 is predominantly expressed in the small intestine and colon, but not in normal esophageal squamous epithelia or gastric epithelia. CDX2 expression is observed in the intestinal metastasis area. 701 Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma Peng D, El-Rifai W cell cycle, it must localize in the nucleus. However, in approximately 50% of high-grade dysplasia, p27 was observed in cytoplasmic localization, which renders it inactive. Protein A tumor suppressor protein essential in cell cycle regulation, a CDK inhibitor. Protein Member of the caudal-related homeobox transcription factor gene family. The encoded protein regulates intestine-specific gene expression and differentiation of intestine. It is suggested that CDX2 is a "master switch" gene whose normal expression determines the proximal and distal specialization of the gut in embryogenesis. GPX3 CDKN2A (p16) Location 5q33.1 Note GPX3 is one of the glutathione peroxidase family members, which functions in the detoxification of hydrogen peroxide. Frequent GPX3 gene promoter hypermethylation has recently been demonstrated in Barrett's adenocarcinomas and its precancerous lesions, Barrett's esophagus and dysplasia, and was significantly associated with gene down-regulation. It is noted that GPX3 has been recently reported as a potential tumor suppressor in prostatic carcinomas. Protein GPX3 is a secretory protein. Location 9p21.3 Note Tumor suppressor gene controls the G1/S transition of the cell cycle. Inactivation of CDKN2A is among the most common genetic/epigenetic alterations through Barrett's carcinogenesis and is an early event. LOH, promoter hypermethylation, or sequence mutations have been reported in over 85% of Barrett's esophagus that were associated with p16 inactivation. DNA / RNA 7288 bp. Exon Count: 3. Protein Tumor suppressor protein having 156 aa, functions as an inhibitor of CDK4 kinase in cell cycle G1 control. GPX7 Location 1p32.3 Note GPX7 is one of the glutathione peroxidase family members which function in the detoxification of hydrogen peroxide. Unlike other glutathione peroxidase family members, GPX7 incorporates cysteine instead of selenocysteine in the conserved catalytic motif. Frequent GPX7 gene promoter hypermethylation has recently been demonstrated in Barrett's adenocarcinomas and its precancerous lesion, Barrett's dysplasia, and was significantly associated with gene down-regulation. Recent research indicates that GPX7 may have dual functions, antioxidative activity and tumor suppressor function in Barrett's adenocarcinoma. DNA / RNA Genomic Size: 6679. Exon Count: 3. Coding Exon Count: 3. TP53 Location 17p13 Note Loss of p53 occurs through either LOH, sequence mutation, or both. Loss of p53 has been reported in Barrett's esophagus and likely correlates with progression to adenocarcinoma, as patients with LOH in TP53 are 16 times more likely to progress to adenocarcinoma than patients without TP53 LOH. DNA / RNA Exon Count: 11. Protein A tumor suppressor protein essential in cell cycle regulation and in DNA damage repair. p53 transcriptionally regulates multiple genes functioning as an inhibitor of cell growth and proliferation and inducer of apoptosis. Loss of TP53 functions promotes tumor progression, most likely by preventing cell cycle arrest, suppressing apoptosis and permitting genetic instability for subsequent genetic alterations. MGMT Location 10q26.3 Note It is recently reported that hypermethylation was detected in 78.9% of esophageal adenocarcinomas, in 100% of Barrett's intraepithelial neoplasia, in 88.9% of Barrett's metaplasia, in only 21.4% of normal esophageal mucosa samples (P<0.001), and correlated significantly with the down-regulation of MGMT transcripts (P=0.048) and protein expression (P=0.02). The decrease of protein expression was significantly CDKN1B (p27) Location 12p13 Note It has been reported that in 83% of Barrett's adenocarcinomas, p27 protein was down-regulated by an immunohistochemical study. And for p27 to arrest Atlas Genet Cytogenet Oncol Haematol. 2010; 14(7) 702 Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma Peng D, El-Rifai W correlated with progressed stage of disease, lymph node invasion and tumor size. DNA / RNA Genomic Size: 299903. Exon Count: 5. Coding Exon Count: 4. Protein O6-methylguanine-DNA methyltransferase is involved in the cellular defense against the biological effects of O6-methylguanine (O6-MeG) in DNA. It repairs alkylated guanine in DNA by stoichiometrically transferring the alkyl group at the O6 position to a cysteine residue in the enzyme. References BARRETT NR. The lower esophagus lined by columnar epithelium. Surgery. 1957 Jun;41(6):881-94 El-Rifai W, Frierson HF Jr, Moskaluk CA, Harper JC, Petroni GR, Bissonette EA, Jones DR, Knuutila S, Powell SM. Genetic differences between adenocarcinomas arising in Barrett's esophagus and gastric mucosa. Gastroenterology. 2001 Sep;121(3):592-8 Bian YS, Osterheld MC, Fontolliet C, Bosman FT, Benhattar J. p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett's esophagus. Gastroenterology. 2002 Apr;122(4):1113-21 Koppert LB, Wijnhoven BP, van Dekken H, Tilanus HW, Dinjens WN. The molecular biology of esophageal adenocarcinoma. J Surg Oncol. 2005 Dec 1;92(3):169-90 APC Lee OJ, Schneider-Stock R, McChesney PA, Kuester D, Roessner A, Vieth M, Moskaluk CA, El-Rifai W. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett's tumorigenesis. Neoplasia. 2005 Sep;7(9):854-61 Location 5q21 Note LOH of 5q has been reported as a higher occurrence in high-grade dysplasia and adenocarcinomas of the esophagus. Hypermethylation of the APC gene has been found in 68-100% of Barrett's adenocarcinomas and approximately 50% of Barrett's esophagus. More importantly, hypermethylation of APC with other genes such as p16, strongly predicts progression to high-grade dysplasia or cancer in patients with BE. Absence of p16 and APC hypermethylation is associated with a benign course. DNA / RNA Genomic Size: 138719. Exon Count: 16. Coding Exon Count: 15. Protein Adenomatous polyposis coli protein which possesses tumor suppressor functions, works as an antagonist of the Wnt signaling pathway. APC binding to beta catenin leads to ubiquitin-mediated beta catenin destruction; loss of APC function increases transcription of beta catenin targets, such as C-MYC and Cyclin D. It is also involved in other processes including cell migration and adhesion, transcriptional activation, and apoptosis. Germline defects in this gene cause familial adenomatous polyposis (FAP), an autosomal dominant pre-malignant disease that usually progresses to malignancy. Disease-associated mutations tend to be clustered in a small region designated the mutation cluster region (MCR) and result in a truncated protein product. Atlas Genet Cytogenet Oncol Haematol. 2010; 14(7) Oberg S, Wenner J, Johansson J, Walther B, Willén R. Barrett esophagus: risk factors for progression to dysplasia and adenocarcinoma. Ann Surg. 2005 Jul;242(1):49-54 Wong A, Fitzgerald RC. Epidemiologic risk factors for Barrett's esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol. 2005 Jan;3(1):1-10 Fitzgerald RC. Molecular basis of Barrett's oesophagus and oesophageal adenocarcinoma. Gut. 2006 Dec;55(12):1810-20 Maley CC. Multistage carcinogenesis in Barrett's esophagus. Cancer Lett. 2007 Jan 8;245(1-2):22-32 Razvi MH, Peng D, Dar AA, Powell SM, Frierson HF Jr, Moskaluk CA, Washington K, El-Rifai W. Transcriptional oncogenomic hot spots in Barrett's adenocarcinomas: serial analysis of gene expression. Genes Chromosomes Cancer. 2007 Oct;46(10):914-28 Kuester D, El-Rifai W, Peng D, Ruemmele P, Kroeckel I, Peters B, Moskaluk CA, Stolte M, Mönkemüller K, Meyer F, Schulz HU, Hartmann A, Roessner A, Schneider-Stock R. Silencing of MGMT expression by promoter hypermethylation in the metaplasia-dysplasia-carcinoma sequence of Barrett's esophagus. Cancer Lett. 2009 Mar 8;275(1):117-26 Peng DF, Razvi M, Chen H, Washington K, Roessner A, Schneider-Stock R, El-Rifai W. DNA hypermethylation regulates the expression of members of the Mu-class glutathione S-transferases and glutathione peroxidases in Barrett's adenocarcinoma. Gut. 2009 Jan;58(1):5-15 This article should be referenced as such: Peng D, El-Rifai W. Esophagus: Barrett's esophagus, dysplasia and adenocarcinoma. Atlas Genet Cytogenet Oncol Haematol. 2010; 14(7):698-703. 703