* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Archives of Microbiology
Survey
Document related concepts
Extracellular matrix wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Tissue engineering wikipedia , lookup
Signal transduction wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cellular differentiation wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell encapsulation wikipedia , lookup
Transcript
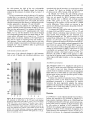
Arch Microbiol (1994) 161:310-315 Archives of Hicrobiolegy © SpringerVerlag 1994 Several phenotypic changes in the cell envelope of Agrobacterium tumefaciens chvB mutants are prevented by calcium limitation Saskia Swart, Trudy J. J. Logman, Ben J. J. Lugtenberg, Gerrit Smit, Jan W. Kijne Institute of Molecular Plant Sciences, Leiden University, P.O. Box 9505, 2333 AL Leiden, The Netherlands Received: 24 May 1993/Accepted: 8 October 1993 Abstract. The chvB gene of Agrobacterium tumefaciens encodes a 235 k D a proteinaceous intermediate involved in the synthesis of/%1,2-glucan, chvB mutants show a pleiotropic phenotype. Besides not to produce cyclic /?-l,2-glucan, chvB mutants have been reported to be avirulent, attachment-deficient, and nonmotile. In this study we report additional differences from the parent strain, p r o b a b l y all linked to changes in the cell envelope. This pleiotropic phenotype - except for attachment and virulence - could largely be prevented by growing chvB cells with low levels of calcium. Although a role for fi-l,2-glucan in o s m o a d a p t a t i o n has been proposed, the m o d e of action of/3-1,2-glucan is not known. We speculate that in A. tumefaciens fl-l,2-glucan stabilizes membranes, which would be important especially in hypotonie media containing calcium. Key words: Agrobacterium - f l - 1 , 2 - G l u c a n - chvB Mut a n t s - Ca 2 + chvB is one of the c h r o m o s o m a l genes of Agrobacterium tumefaciens usually required for transformation of plants (Douglas et al. 1982, 1985). The chvB gene is required for the synthesis of a low molecular weight cyclic fi-l,2glucan. The gene encodes a 235 k D a cytoplasmic m e m brane protein, which is an intermediate in fl-l,2-glucan synthesis (Zorreguieta and Ugalde 1986). Another virulence gene, chvA, is required for transport of fi-l,2-glucan to the periplasmic space (Cangelosi et al. 1989). However, chvB mutants are not only impaired in fi-l,2-glucan production. They also exhibit reduced motility and resistance to certain phages, due to absence of flagella (Bradley et al. 1984), and are attachment-deficient and Abbreviations: Cb, carbenicillin; Km, kanamycin; TCA, trichloroacetic acid; Kay fraction of the stationary gel volume available for diffusion; LPS, lipopolysaccharide; SDS-PAGE, Sodium dodecyl sulphate polyacrylamide gel electrophoresis Correspondence to: S. Swart, Mogen International, Einsteinweg 97, 2333 CB Leiden, The Netherlands Tel. (0) 71 25 82 82; Fax (0) 71 22 1471 avirulent (Douglas et al. 1982, 1985). A large part of the chvB gene is not required for glucan synthesis. Also attachment ability and virulence are not impaired by deletion of this part of the chvB gene, suggesting that the latter properties are coupled. Since wild-type A. tumefaciens cells accumulate fl-l,2-glucan in the periplasmic space when grown under hypo-osmotic conditions, synthesis of cyclic fi- 1,2-glucan appears to be osmoregulated (Miller et al. 1986). Cangelosi et al. (1990) reported for chvB mutants a growth rate lower than that of wild-type cells and an altered periplasmic and cytoplasmic protein content when mutant cells were cultured in low-osmoticstrength media, but not for cells cultured in high-osmoticstrength media. These observations support a role for periplasmic glucan in osmoadaptation. Inadequate osm o a d a p t a t i o n m a y result in deficiencies of the cell envelope. However, the mechanism of action of fi-l,2glucan is not known. This study shows that the pleiotropic phenotype of chvB mutants of A. tumefaciens can largely be prevented by growing chvB cells with low levels of calcium. We speculate that fl-l,2-glucan is involved in stabilization of membranes, especially in hypo-osmotic media containing calcium. Materials and methods Bacterial strains and culture conditions Agrobacterium tumefaciens strains and mutants were generous gifts from E. W. Nester (University of Washington, Seattle, Wash., USA), and have been described by Douglas et al. (1985). A. tumefaciens strain A348 has a wild-type C58 chromosome and harbours plasmid pTiA6NC (Garfinkel et al. 1981). A348 has a wild-type phenotype. chvA mutants ME42 and ME66 and chvB mutants MEll7 and ME 118 have a Tn3Hoho 1 insertion, including a Cb resistance gene, in the chromosomal virulence A (chvA) or B (chvB) gene, respectively, chvB mutants A1011 and A1045 have a Tn5 insertion, including a Km resistance gene, in the chvB gene. Bacteria were grown at 28 °C in 100-ml Erlenmeyer flasks containing 50ml medium on a rotary shaker (180 rpm). The composition of TY medium was 0.5% tryptone-0.3% yeast extract. According to the manufacturer (Difco Laboratories, Detroit, Mich., USA), the Ca 2÷ concentration of this medium is 0.15 mM and the Mg 2+ concentra- 311 tion is 0.13 mM. TYC medium is TY medium supplemented with 7 mM CaClz (Beringer 1974). Swarm plates contain in addition 0.3% (w/v) agar. Plant culture conditions Seeds of pea (Pisum sativum cv. Finale, purchased from Cebeco, Rotterdam, The Netherlands) were surface-sterilized and cultivated as previously described (Smit et al. 1986). Kalanchob"daigremontiana plants were cultivated as described by Ooms et al. (1980). Measurement of medium osmolarity The osmolarity of media was measured with an osmomat 030 cryoscopic osmometer (Salm and Kipp, Breukelen, The Netherlands). Results Growth Attachment assay The attachment assay used was previously described by Smit et al. (1986). Briefly, bacteria were grown to early stationary phase, centrifuged and resuspended in 25 mM potassium phosphate buffer, pH 7.5, to 3 x 108 bacteria per nal. After incubation of lateral roots from 7-day-old axenically grown pea plants for 2 h in this suspension, the roots were washed in phosphate buffer and screened for attached bacteria by phase-contrast microscopy. In this study attachment to root hairs was ranked into three classes: class 1, no attached bacteria; class 2, few bacteria directly attached to the root hair; class 3, many attached bacteria forming two or more layers or a caplike aggregate on top of the root hair. The variability of the test was about 10% and depended largely on the condition of the roots. ldrulence assays In the greenhouse, leaves of K. daigremontiana plants were wounded with a toothpick. Cells of early stationary phase cultures of A. tumefaciens strain A348 or strain MEll7, grown in TYC or TY medium, were harvested and resuspended in phosphate buffer to an A6z0 value of 1.0. Ten gl of this suspension were added per wound site. Tumour formation was quantified three weeks after inoculation. Purification of flagella Flagella were isolated from the bacterial cell surface and purified by sucrose gradient ultracentrifugation according to the method of Carsiotis et al. (1984). Isolation of cell envelope fractions and lipopolysaccharide Bacteria were grown to early stationary phase. Cell membrane preparations were made according to De Maagd et al. (1989). Proteins were separated on 11% polyacrylamide gels as described by Lugtenberg et al. (1975). For analysis of LPS, cell envelope preparations in sample buffer, each containing an equal amount of protein, were treated first with proteinase K (0.2 mg/ml) for 60 min at 60 °C and then applied to a 15% SDS-polyacrylamide gel. Proteins and LPS were visualized by silver-staining (Blum et al. 1987). Purification of fl-l,2-glucan To test the production of fl-1,2-glucan, cells from 11 early stationary phase cultures were harvested by centrifugation at 10,000 x g for 10 min. Pellets were extracted with 1% TCA for 30 min at room temperature. TCA extracts were neutralized, concentrated, and applied to a Sephadex G50 column (63.5 x0.8 cm), which was subsequently eluted with 7% propanol. One ml fractions were tested for the presence of carbohydrates by using the phenol-sulphuric acid method (Hanson and Philips 1981). The saccharide composition of these carbohydrate fractions was determined by gas-liquid chromatography after sample hydrolysis (Albersheim et al. 1967) (liquid phase: 3% ECNSS/M on GasChromQ [Applied Science, Oud-Beijerland, The Netherlands]). Agrobacterium tumefaciens strain A348, g r o w n in the s t a n d a r d g r o w t h m e d i u m TYC, h a d a doubling time of a b o u t 2.1 h, reaching a final A620-value of 1.2 to 1.3. As shown in Table 1, omission of the calcium supplement (TY medium) yielded for this strain the same g r o w t h characteristics as seen in T Y C medium. The osmolarity of TY and T Y C media is 0.043 and 0.065 osM, respectively. Because chvB m u t a n t s have been reported to be impaired in g r o w t h in low osmolarity m e d i u m (Cangelosi et al. 1990), we tested whether chvB m u t a n t s grew slower in TY c o m p a r e d to T Y C media. However, chvB m u t a n t strain M E l l 7 appeared to grow slower in T Y C with a doubling time of 3.4 h, reaching a final A6zo-value of 0.8 to 0.9, whereas the doubling time and the g r o w t h m a x i m u m a p p e a r e d to be restored to nearly the level of strain A348 by omitting the calcium supplement from the m e d i u m (Table 1). Similar results were obtained with chvB m u t a n t s A1011, A1045, and M E l l 8 , and chvA m u t a n t s M E 4 2 and M E 6 6 (data n o t shown). Strain M E l l 7 h a r b o u r s a T n 3 H o H o l t r a n s p o s o n in chvB carrying a Cb resistance gene. Despite the presence of this gene, M E l l 7 does n o t grow in T Y C m e d i u m supplemented with 100 gg/ml Cb (Table 1). In contrast, in TY m e d i u m M E l l 7 grows n o r m a l l y in the presence of Cb, indicative of expression of the Cb resistance gene. Several other chvB strains were tested for this phenotype. M E l l 8 also h a r b o u r e d a C b resistance gene, and appeared to be Cb-resistant only when g r o w n in TY medium. Similar results were obtained with chvA m u t a n t s M E 4 2 and M E 6 6 (data not shown). The other chvB strains studied, A1011 and A1045, are T n 5 - m u t a n t s thus harb o u r i n g a K m resistance gene. P h e n o t y p i c expression of K m resistance appeared not to depend on the calcium concentration of TY m e d i u m (data not shown). Cells of strain A348 are not Cb resistant and did grow neither in TY nor in T Y C media supplemented with 100 gg/ml Cb. W h e n the Cb concentration was lowered to 10 gg/ml Cb, cells of strain A348 showed an impaired g r o w t h in b o t h TY and T Y C media, being only slightly m o r e impaired in T Y C medium. Production of fl- l,2-glucan W h e n strain A348 was tested for p r o d u c t i o n of fl-l,2glucan using gel permeation c h r o m a t o g r a p h y , a c a r b o h y drate-positive peak was observed at a Kay of 0.6, corresp o n d i n g with the Kav previously found for cyclic/~-l,2glucan (Cangelosi et al. 1989; Miller et al. 1986). The a m o u n t of cell-associated soluble c a r b o h y d r a t e with a Kay of 0.6 p r o d u c e d by A348 in 1 1 T Y C m e d i u m (5 g 312 Table 1. Characteristics of Agrobacterium tumefaciens strain A348 and its chvB mutant strain ME117 grown in TY or TYC a media Strain Growth b medium Final A62ovalue Doubling time, h Cb r~ Motility Attachment Virulence A348 TYC TY TYC TY 1.2 1.3 1.2-1.3 0.8-0.9 1.1 - 1.2 2.1 2.1 3.4 2.5 . + + + MEll7 (chvB) + + . . + + -. - - a TY is TYC medium (0.5% tryptone 0.3% yeast extract-7 mM CaC12)without the calcium supplement u Determined by the method of Smit et al. (1986). More than 80% class 1 was designated "no attachment" Growth in TY or TYC media supplemented with 100 gg/ml Cb fresh weight of cells) was 31 _+ 3 mg. However, from bacteria grown in TY medium only 11 _+ 2 mg of carbohydrate with a Kay of 0.6 could be isolated. The saccharide composition of both carbohydrate fractions, as determined by gas-liquid chromatography, appeared to be identical, with glucose representing the major saccharide detected. Next production of fi-l,2-glucan by strain ME117 grown in T Y C or TY medium was tested. As judged from the a m o u n t of carbohydrate-positive material detected, ME117 does not produce fi-l,2-glucan when grown in either TY or T Y C media. In view of the possibility that fi-1,2-glucan was excreted, the growth media were chekked for the presence of fi-l,2-glucan. Extracellular cyclic /?-1,2 glucan was found neither with the parent strain nor with chvB cultures. chvB mutants have been reported to show a reduced n u m b e r of flagella (Bradley et al. 1984). Electron microscopic observations showed that the number of flagella on the surface of ME117 increased significantly when the bacteria were grown in TY medium (data not shown). We c o m p a r e d flagella preparations of A348 and ME117, grown either in TY or TYC, by S D S - P A G E analysis (Fig. 1 A). S D S - P A G E of purified flagella of A. tumefaciens strain A348 showed two polypeptides of 30 k D a and 32 kDa, respectively (Fig. 1A, lanes 1 and 2). At present it is unknown whether the band with higher mobility represents an artefact, e.g., a proteolytic product of flagellin, or whether the two bands correspond to two isotypes of flagellin, the m a j o r protein of a flagellum. F o r Motility Parent strain A348 is motile during exponential growth, in both TY and T Y C media. Only in the late stationary phase the cells become nonmotile. In contrast, cells of strain ME117 are nonmotile when grown in T Y C medium, as judged from phase-contrast microscopy (data not shown). However, when grown in TY medium M E l l 7 cells are motile, although not to the same extent as are cells of strain A348. Motility was also assessed by using swarm plates. On TY swarm plates cells of strain ME117 formed swarms, although smaller than did cells of the parent strain. On T Y C plates m u t a n t cells formed only a small colony, confined exclusively to the site of inoculation, whereas A348 cells formed swarms as large as those seen on TY swarm plates (Fig. 1B). The same observations were made for the other chvB mutants tested, A1011, A1045 and ME118. Also the chvA mutants ME42 and ME66 showed an increase in mobility when grown in TY c o m p a r e d to T Y C (data not shown). However, for these mutants the difference was smaller, chvA mutants grown in T Y C were more motile than chvB mutants grown in TYC, whereas the motility of both strains grown in TY was similar, chvA mutants have been reported to have a leaky phenotype. The mutants still export 2% of the wild-type a m o u n t of fi-l,2-glucan (O'Connell and H a n d e l s m a n 1989). Moreover, chvA mutants occasionally form small tumours, and when harbouring a pSym9 plasmid, can form small root nodules (Van Veen et al. 1987). Therefore, we decided not to further use chvA mutants in this study. A B kDa 97-66-45-36-29-- 18-- 12345 Fig. 1A. Silver-stained SDS-PAGE (15%) profiles of crude flagella preparations of Agrobacterium tumefaciens strain A348 (lanes 1 and 2) and chvB mutant strain MEl17 (lanes 3, 4 and 5) grown in TY medium (lanes 1 and 3) or TY supplemented with 7 mM CaC12 (TYC) (lanes 2, 4 and 5). The amount applied corresponds with flagella of 7 x 108 bacteria, with the exception of lane 5 (1.4 x 101° bacteria). Positions of molecular weight markers are indicated at the left; B 0.3 % agar swarm-plates containing TYC (uppear plate) or TY (lower plate) medium spotted with A348 (1 and 3) and ME117 (2 and 4) 313 the chvB mutant the yield of the two polypeptides corresponding with the flagellin bands was strongly reduced in the presence of calcium (Fig. 1 A, lanes 3, 4 and 5). Use of a concentration series of calcium in TY medium revealed that at an amount of between 2.0 and 2.5 m M calcium added, the mutant cells became nonmotile. Since the calcium concentration in TY is 0.15 mM, the critical calcium concentration that directly or indirectly prevents motility apparently is between 2.15 and 2.65 raM. Next to calcium the addition of 7 m M MgC12 to TY medium reduced growth and motility of ME117, but the effect was less pronounced. For instance, the final A620 of M E l l 7 cells grown in TY supplemented with 7 m M MgC12 was 1.1 and the diameter of the colony formed on corresponding swarm plates was larger than on TYC swarm plates, although not as large as on TY swarm plates (data not shown). Addition of 10.5 m M NaC1 or KC1 did not result in reduced growth and motility of chvB mutants. These results indicate that not just salt, but rather a higher concentration of divalent cations and especially Ca 2 ÷ has an inhibitory effect on growth and motility of chvB mutants. Cell envelope proteins and L P S Since some of the observed changes in chvB mutants, such as lack of flagella and attachment-deficiency, were A B associated with the cell envelope, we examined the effect of reduced Ca2+-levels on profiles of cell envelope proteins and the LPS of strains A348 and M E l l 7 . At reduced Ca2+-levels, the cell envelope protein profiles of the bacteria are altered. However, this alteration was not specific for M E l l 7 bacteria, since also A348 bacteria show a different cell envelope protein profile when grown in TY and TYC. Nevertheless, comparison of the profiles of A348 and M E l l 7 yielded several differences. These include an increase in the amount of a 20 kDa protein band in the profile of ME117 grown in TYC (Fig. 2A). To compare the LPS profiles and the amount of LPS of strains A348 and M E l l 7 grown in TYC or TY, cell envelope isolates containing equal amounts of protein were treated with proteinase K and separated in a SDS-polyacrylamide gel. The relative amount of LPS isolated from A348 grown in TYC was smaller than the amount of LPS from A348 grown in TY (Fig. 2B). However, whereas the amount of LPS from strain ME117 grown in TY was only slightly reduced compared to that from A348, the amount of LPS from M E l l 7 grown in TYC was severely reduced (Fig. 2B). Since the protein content of cell envelopes of ME117 cells grown in TYC is probably not increased, M E l l 7 cells grown in TYC apparently contain a decreased amount of LPS. Furthermore, the profile of M E l l 7 LPS appears to differ from that of the parent strain in that the banding pattern of the region with higher motility, i.e. the core region, is different. Attachment and virulence kDa The attach.ment ability of A. tumefaciens cells grown in TYC and TY media was tested. Wild-type cells grown in TYC attach to pea root hairs. However, omission of calcium from the medium resulted in loss of attachment ability (Smit et al. 1987) (Table 1). Cells of strain M E l l 7 did not attach to pea root hair tips when grown in either TYC or TY media (Table 1). Virulence was tested on KalanchoY leaves. Wild-type cells grown in TYC or TY media form tumours. Since attachment presumably is a prerequisite for tumour formation, the plant apparently supplies the calcium needed for the attachment of wild-type cells grown in TY medium. Cells of chvB strain M E l l 7 grown in TYC or TY media were avirulent (Table 1). 66-- 45-36-29-- 18-14-- Discussion 1 2 3 4 1 2 3 4 Fig. 2A, B. Silver-stained ceil envelope profiles of A. tumefaciens cells separated by SDS-PAGE. A An 11% gel of ceil envelope proteins of chvB mutant strain ME117 (lanes 1 and 2) and parent strain A348 (lanes 3 and 4) grown in TY medium (lanes 1 and 3) or TY supplemented with 7 mM CaC12 (TYC) (lanes 2 and 4). Positions of molecular weight markers are indicated at the left. An arrow head indicates the position of the 20-kDa protein. B LPS profiles. Cell envelope isolations containing an equal amount of protein were treated with proteinase K and applied to a 15% gel. Lanes are numbered as in A fi-l,2-Glucan is a cyclic oligosaccharide found in Rhizobiaceae and reported to be involved in adaptation to hypo-osmotic conditions. Miller et al. (1986) found less fi-l,2-glucan produced by wild-type Agrobacterium tumefaciens cells grown in medium of high osmolarity than by cells grown in medium of low osmolarity. Furthermore, fi-l,2-glucan negative mutants are impaired in growth in medium of low osmolarity (Cangelosi et al. 1990). However, the mechanism of action of cyclic fl-l,2glucan is not known. In a hypotonic environment cells 314 have to deal with an influx of water which causes swelling of the cells. Gram-negative bacteria have a relatively rigid cell wall, but when the influx of water proceeds and the cell wall cannot extend further, rupture due to a process of brittle fracture will occur (Corner and Marquis 1969). It has been reported that sugars protect membrane structures from damage, for instance during freeze-thaw cycles (Crowe et al. 1984a; Rudolph and Crowe 1985). Furthermore, several reports point out interactions between carbohydrates and phospholipid monolayers. Carbohydrates would coordinate to the polar headgroups of the lipidlayer by hydrogen bonds and in doing so alter their mobility and/or packing density thereby increasing the film area (Crowe et al. 1984b; Johnston et al. 1984). We speculate that cyclic fi-l,2-glucan is involved in stabilizing the lipid bilayers of the cytoplasmic and outer membranes of Rhizobiaceae grown in hypotonic media. Calcium is known to adsorb onto membrane surfaces causing disruption of the membrane and by that the cell, especially in aqueous solutions (BfikAs and Disalvo 1991; Lis et al. 1981). A disruption of cell envelope membranes would result in impaired assembly of membrane structures such as LPS and flagella. This offers a possible explanation for the toxicity of calcium for fl-l,2-glucannegative mutants, as described in this study. Apparently fi-l,2-glucan-negative mutants are very sensitive to high calcium concentrations. The pleiotropic phenotype of chvB mutants, i.e. attachment inability, avirulence, lack of flagella, altered cell envelope profile and altered LPS profile, mainly corresponds to changes in the cell envelope. A chvB mutant seems to show impaired assembly of outer membrane components when grown in TYC medium. If periplasmic fl-l,2-glucan can stabilize membranes and may compete for Ca 2 +-binding sites at polar headgroups, a fi-l,2-glucan-eontaining cell would have less problems with disruption by calcium than would have a fi-l,2-glucan-negative mutant. We found two indications for a correlation between fi-l,2-glucan and calcium: (i) A348 bacteria produce two to three times more fi-l,2-glucan when grown in TYC medium than do cells grown in TY medium, despite the fact that TYC medium has a higher osmolarity than TY medium; this suggests that the osmolarity of the medium is one but not the only factor influencing the production of fl-l,2glucan. (ii) Omission of calcium from the medium restored the growth of chvB mutant M E l l 7 to nearly wild-type levels and allowed growth of M E l l 7 in 100 gg/ml Cb. Moreover, these bacteria became motile in TY and the production of flagella was restored. Also, the cell envelope profiles and the amount of LPS were more like those of the parent strain when the mutant bacteria were grown in TY (although also the parent strain cells showed a difference in these characteristics when grown in TY versus TYC medium). Attachment and virulence were not restored when calcium was omitted from TYC medium. This suggests that (i) fi-l,2-glucan could be directly involved in attachment; although this seems unlikely, however, since exogenously added fi-l,2-glucan does not affect attachment (O'Connell and Handelsman 1989; S. Swart, unpublished results), (ii) fl-l,2-glucan is needed for the synthesis or functioning of an attachment factor, or (iii) calcium is needed for virulence and/or attachment. The calciumbinding protein rhicadhesin, which is involved in the first step of attachment of Rhizobiaceae to plant cells (Smit et al. 1989) has been reported to be anchored to the bacterial cell surface in a calcium-dependent manner (Smit et al. 1991). As a hypothesis, we propose that above a critical level (between 2.15 and 2.65 raM) calcium is toxic to fi-l,2-glucan deficient mutants but that, on the other hand, calcium is required for anchoring, stabilization and/or activation of rhicadhesin. Absence of fi-l,2glucan apparently results in deregulation of this process. Ongoing research is focused on the role of rhicadhesin in virulence ofA. tumefaciens bacteria and on the possible connection between fi-1,2-glucan, calcium and rhicadhesin. Acknowledgements. This work was supported by the Foundation for Fundamental Biological Research (BION), which is subsidized by the Netherlands Organization of ScientificResearch (NWO). We thank Otto Geiger for stimulating discussions. References Albersheim P, Nevins D, English PD, Karr A (1967) A method for the analysis of sugars in plant cell wall polysaccharides by gas-liquid chromatography. Carbohydr Res 5:340-345 B/tkgts LS, Disalvo EA (1991) Effect on the cryoprotective action of trehalose. Cryobiology 28:347-353 Beringer J (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188-198 Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99 Bradley DE, Douglas CJ, Peschon J (1984) Flagella-specific bacteriophages of Agrobacterium tumefaciens: demonstration of virulence of nonmotile mutants. Can J Microbiol 30:676-681 Cangelosi GA, Martinetti G, Leigh JA, Lee CC, Theines C, Nester EW (1989) Role for Agrobacterium tumefaciens chvA protein in export of fl-l,2-glucan. J Bacteriol 171:1609 1615 Cangelosi GA, Martinetti G, Nester EW (1990) Osmosensitivity phenotypes of Agrobacterium tumefaciens mutants that lack periplasmic fl-l,2-glucan. J Bacteriol 172:2172 2174 Carsiotis M, Weinstein DL, Karch H, Holder IA, O'Brien AD (1984) Flagella of Salmonella typhimurium are a virulence factor in infected C57BL/6J mice. Infect Immun 46:814 818 Corner TR, Marquis RE (1969) Why do bacterial protoplasts burst in hypotonic solutions? Biochim Biophys Acta 183:544-558 Crowe LM, Mourdian R, Crowe JH, Jackson SA, Womeisly C (1984a) Effect of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta 769:141-150 Crowe JH, Whittam MA, Chapmen D, Crowe LM (1984b) Interactions of phospholipid monolayers with carbohydrates. Biochim Biophys Acta 769:151-159 De Maagd RA, Rijk R de, Mulders IHM, Lutgenberg BJJ (1989) Immunological characterization of Rhizobium leguminosarum outer membrane antigens using polyclonal and monoclonal antibodies. J Bacteriol 171:1136 1142 Douglas CJ, Halperin W, Nester EW (1982) Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol 152:1265-1275 Douglas CJ, Staneloni RJ, Rubin RA, Nester EW (1985) Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol 161:850-860 Garfinkel D J, Simpson RB, Ream LW, White FF, Gordon MP, Nester EW (1981) Genetic analysis of crown gall: fine structure 315 map of the T-DNA by site-directed mutagenesis. Cell 27: 143-153 Hanson RS, Philips JA (1981) Chemical composition. In: Gerhardt P, Murray RGE, Costilow RN, Nester EW, Wood WA, Krieg NR, Philips GB (eds) Manual of methods for general bacteriology. American Society for Microbiology, Washington DC, pp 328-364 Johnston DS, Coppard E, Parera PV, Chapman D (1984) Langmuir film balance study of the interaction between carbohydrates and phospholipid monotayers. Biochemistry 23:6912 6919 Lis LJ, Parsegian VA, Rand RP (1981) Binding of divalent cations to dipalmitoylphosphatidylcholine bilayers and its effect on bilayer interaction. Biochemistry 20:1761-1770 Lugtenberg BJJ, Meyers J, Peters R, Hoek P van der, Alphen L van (1975) Electrophoretic resolution of the major outer membrane protein of Escherichia coli K12 into four bands. FEBS Lett 58:254-258 Miller KJ, Kennedy EP, Reinhold VN (1986) Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science 231:48 51 O'Connetl KP, Handelsman J (1989) ChvA locus may be involved in export of neutral cyclic /~-l,2-1inked glucan from Agrobacterium tumefaciens. Mol Plant-Microbe Int 2:11 16 Ooms G, Hooykaas PJJ, Poulis JA, Schitperoort RA (1980) Characterization of Tn904 insertions in octopine Ti plasmid mutants of Agrobacterium tumefaciens. J Bacteriol 144:82 91 Rudolph A, Crowe JH (1985) Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 22:367 377 Smit G, Kijne JW, Lugtenberg BJJ (1986) Correlation between extracellular fibrils and attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol 168:821-827 Smit G, Kijne JW, Lugtenberg BJJ (1987) Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol 169:4294-4301 Smit G, Logman TJJ, Boerrigter METI, Kijne JW, Lugtenberg BJJ (1989) Purification and partial characterization of the Rhizobium leguminosarum biovar viciae Ca2+-dependent adhesin which mediates the first step in attachment of cells of the family Rhizobiaceae to plant root hair tips. J Bacteriol 171:4054-4062 Smit G, Tubbing DMJ, Kijne JW, Lugtenberg BJJ (1991) Role of Ca 2+ in the activity of rhicadhesin from Rhizobium Ieguminosarum biovar viciae, which mediates the first step in attachment of Rhizobiaceae cells to plant root hair tips. Arch Microbiol 155:278 283 Van Veen RJH, Den Dulk-Ras H, Schilperoort RA, Hooykaas PJJ (1987) Chromosomal nodulation genes: Sym-plasmid containing Agrobacterium strains need chromosomal virulence genes (chvA and chvB) for nodulation. Plant Mol Biol 8:105-108 Zorreguieta A, Ugalde RA (1986) Formation in Rhizobium and Agrobaaerium spp. of a 235 kDa protein intermediate in /~D(1,2)-glucan synthesis. J Bacteriol 167:947 951