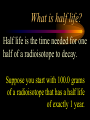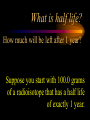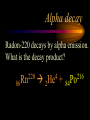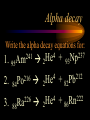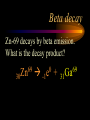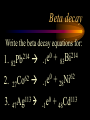* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nuclear Chemistry
Survey
Document related concepts
Nuclear fission wikipedia , lookup
Nuclear fission product wikipedia , lookup
Background radiation wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Ionizing radiation wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear drip line wikipedia , lookup
Valley of stability wikipedia , lookup
Transcript
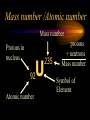
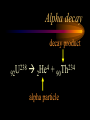
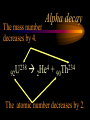
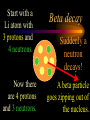
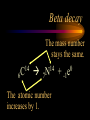
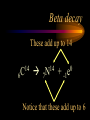
Nuclear Chemistry Only one element has unique names for its isotopes … 1 1H hydrogen 2 H 1 deuterium 3 H 1 tritium Deuterium and tritium are used in nuclear reactors and fusion research. Some isotopes are radioactive Radioactive isotopes are called radioisotopes. Radioisotopes can emit alpha, beta or gamma radiation as they decay. Man-made Isotopes Man-made isotopes are usually made by bombarding atoms with protons or neutrons. Cobalt-59 occurs naturally. When a neutron “sticks” to the nucleus, cobalt-60 is formed. Uses for Isotopes Radioisotopes are used to kill cancer cells. (Co-60, Bi-212) Radioisotopes are used in “imaging” living and nonliving systems. Radioisotopes are used as tracers in chemical reactions. Properties of alpha, beta and gamma radiation Subatomic particles 1 0 proton 1 1 H n neutron electron e -1 0 What do the numbers represent? Mass number /Atomic number Mass number Protons in nucleus U 92 Atomic number 235 protons + neutrons Mass number Symbol of Element Alpha (a) particles are the nuclei of helium atoms 4 and have the symbol 2He . What is the atomic number of an a particle? 2 4 He Alpha (a) particles are the nuclei of helium atoms 4 and have the symbol 2He . How many times heavier is an alpha particle than a hydrogen atom? 4 Beta (b) particles are high speed electrons ejected from the nuclei of atoms and have 0 the symbol -1e . What is the mass number of a b particle? -1 0 e Beta (b) particles are high speed electrons ejected from the nuclei of atoms and have 0 the symbol -1e . No protons or neutrons in an electron. -1 0 e Beta (b) particles are high speed electrons ejected from the nuclei of atoms and have 0 the symbol -1e . What is the difference between a b particle and None a “regular” electron? Beta (b) particles are high speed electrons ejected from the nuclei of atoms and have 0 the symbol -1e . What is the difference Location between a b particle and Location Location a “regular” electron? Gamma (g) rays are high energy electromagnetic waves, not particles. No protons, neutrons or electrons. Gamma rays have short wavelengths and high energies and travel at the speed of light. Gamma rays have short wavelengths Increasing energy … and high energies. Alpha, Beta, Gamma Electric field from electrically charged plates + + + + + + + + What is the effect of an electric field on a, b, g ? - - - - - - - - Radioactive Source Alpha, Beta, Gamma Electric field from electrically charged plates + + + + + + + + b - - - - - - - - - g a Radioactive Source Alpha, Beta, Gamma Electric field from electrically charged plates b Are a, b and g rays deflected g by magnetic fields? - - - - - - - - a + + + + + + + + Radioactive Source Alpha, Beta, Gamma Paper Lead a Radioactive Source Aluminum foil Alpha, Beta, Gamma Paper Lead b a Radioactive Source Aluminum foil Alpha, Beta, Gamma Paper Lead b a Radioactive Source Aluminum foil g Radiation Project Create a table listing information for each of the three kinds of radiation: Alpha, beta and gamma Properties to include in your table: (1) (2) (3) (4) (5) Greek letter (6) relative mass symbol (7) relative. charge actually is (8) penetrating atomic number ability mass number (9) shielding Nuclear Properties Table Property Alpha Beta Gamma Greek Letter Symbol Actually is… Stop! Atomic number Mass number Relative mass Relative charge Penetrating Shielding Complete the chart on notebook paper, then continue. Nuclear Properties Table Property Greek Letter Symbol Actually is… Atomic number Mass number Relative mass Relative charge Penetrating Shielding Alpha Beta Gamma Nuclear Properties Table Property Greek Letter Symbol Actually is… Atomic number Mass number Relative mass Relative charge Penetrating Shielding Alpha Beta Gamma a b g Nuclear Properties Table Property Greek Letter Symbol Actually is… Atomic number Mass number Relative mass Relative charge Penetrating Shielding Alpha Beta Gamma a b g 4 2He 0 -1e NA Nuclear Properties Table Property Greek Letter Symbol Actually is… Atomic number Mass number Relative mass Relative charge Penetrating Shielding Alpha Beta Gamma a b g 4 2He 0 -1e NA He nucleus electron EM energy Nuclear Properties Table Property Greek Letter Symbol Actually is… Atomic number Mass number Relative mass Relative charge Penetrating Shielding Alpha Beta Gamma a b g 4 2He 0 -1e NA He nucleus electron EM energy 2 -1 NA Nuclear Properties Table Property Alpha Beta Gamma a b g 4 2He 0 -1e NA He nucleus electron EM energy Atomic number 2 -1 NA Mass number 4 0 NA Greek Letter Symbol Actually is… Relative mass Relative charge Penetrating Shielding Nuclear Properties Table Property Alpha Beta Gamma a b g 4 2He 0 -1e NA He nucleus electron EM energy Atomic number 2 -1 NA Mass number 4 0 NA Relative mass 4 1/ 1837 NA Greek Letter Symbol Actually is… Relative charge Penetrating Shielding Nuclear Properties Table Property Alpha Beta Gamma a b g 4 2He 0 -1e NA He nucleus electron EM energy Atomic number 2 -1 NA Mass number 4 0 NA Relative mass 4 1/ 1837 NA +2 -1 NA Greek Letter Symbol Actually is… Relative charge Penetrating Shielding Nuclear Properties Table Property Alpha Beta Gamma a b g 4 2He 0 -1e NA He nucleus electron EM energy Atomic number 2 -1 NA Mass number 4 0 NA Relative mass 4 1/ 1837 NA +2 -1 NA Low Medium High Greek Letter Symbol Actually is… Relative charge Penetrating Shielding Nuclear Properties Table Property Alpha Beta Gamma a b g 4 2He 0 -1e NA He nucleus electron EM energy Atomic number 2 -1 NA Mass number 4 0 NA Relative mass 4 1/ 1837 NA +2 -1 NA Low Medium High Greek Letter Symbol Actually is… Relative charge Penetrating Shielding 2.5 cm of air; Metal, plastic anything else or wood Lead or concrete Protection from radiation 1. Shielding 2. Distance How do you protect yourself from … 2.5 cm of air, paper, skin aluminum, lead, other Beta metals, wood, plastic, etc. Gamma up to a foot or two of lead, many feet of concrete Alpha There are some kinds of radiation you can not protect your self from. Radiation Gamma rays and high energy cosmic particles from space. But there is one kind of radiation hazard that you can protect against. That hazard comes from the uranium beneath your feet. Uranium in the ground decays according to … The uranium decay series Uranium-238 decays through many steps to make stable lead-206 http://library.tedankara.k12.tr/chemistry/vol1/nucchem/trans90.htm The uranium decay series Radon is the only gas in the series. http://library.tedankara.k12.tr/chemistry/vol1/nucchem/trans90.htm Hazards from radon Since radon is the only gas in the decay series of uranium … …it can work its way up through the ground and into your basements and crawl spaces. You breathe radon into your lungs. Hazards from radon And when radon is in your lungs… …it can decay and release an alpha particle … …which travels only a short distance before it is absorbed by your lungs, and transfers its energy. Hazards from radon This ionizing radiation in your lungs can cause lung cancer. Smoking cigarettes and breathing radon really increases your chances of getting lung cancer. Protecting against radon Get a test kit to see if there is a problem. Charcoal canisters, which are sent off for analysis. Abatement: Seal places where gas gets in. Ventilation – bring in fresh air. Half life What is half life? Half life is the time needed for one half of a radioisotope to decay. Suppose you start with 100.0 grams of a radioisotope that has a half life of exactly 1 year. What is half life? How much will be left after 1 year? Suppose you start with 100.0 grams of a radioisotope that has a half life of exactly 1 year. What is half life? After one year there will be 50.0 g left. After a second year there will be 25.0 g left. Suppose you start with 100.0 grams of a radioisotope that has a half life of exactly 1 year. What is half life? After one year there will be 50.0 g left. After a second year there will be 25.0 g left. After a third year there will be 12.5 grams left. After a fourth year there will be 6.25 grams left. Half life project 1. Pick a mass between 10g and 50g. 2. Decide on a half life – any time. 3. Scale your graph – mass on y-axis and at least six (6) half-lives on the x-axis. 4. Plot the masses after intervals of one half-life. Half life project 5. What shape is the graph? 6. When will the mass of the radioisotope fall to zero? 7. When is the radioactivity no longer a problem? 8. What mathematical function describes radioactive decay? Half life project mass 10 5 2.5 time t1/2 t1/2 Half life project mass 10 5 2.5 time t1/2 t1/2 Activity (counts/min) Half life project 200 Exponential decay -kt e A = A0 100 50 t1/2 Time (min) t1/2 Activity (counts/min) 200 100 50 Half life project Radiation is “not a problem” when it falls below background level. background t1/2 Time (min) t1/2 Half life project Questions: 1. A radioisotope has a half-life of 100 years. How long will it take for the radiation to decrease to 1/16 of its original value? 400 years Half life project Questions: 2. A radioisotope has an activity of 560 counts per minute. After 16 hours the count rate has dropped to 35 counts per minute. What is the half life of the radioisotope? 4 hours Decay equations Alpha decay In alpha decay, an alpha particle 4 (2He ) is released from the nucleus. The alpha particle carries away two protons and two neutrons. Alpha decay decay product 238 U 92 2 4 He + 90 234 Th alpha particle The mass number decreases by 4. 238 U 92 2 Alpha decay 4 He 234 Th + 90 The atomic number decreases by 2. Alpha decay These must add up to 238 238 U 92 2 4 He 234 Th + 90 These must add up to 92 Alpha decay Radon-220 decays by alpha emission. What is the decay product? 86 220 Rn 2 4 He + 216 Po 84??? Alpha decay Write the alpha decay equations for: 2 4 He 1. 241 Am 95 2. 216 84Po 4 He 2 3. 226 88Ra 4 He 2 237 Np + 93 + 212 Pb 82 + 86 222 Rn Beta decay Beta decay occurs because of the instability of a neutron. Neutrons are a little more massive than protons; neutrons are neutral. What does this suggest about the composition of neutrons? Beta decay Scientists used to think that neutrons might be a combination of a proton and an electron. We know that neutrons decay into protons, which stay in the nucleus, and electrons, which are ejected from the nucleus as beta particles. Beta decay Decay of a neutron: 1 n 0 neutron 1 H 1 proton + 0 e -1 electron The electron ejected from the nucleus is a beta particle. Beta decay Technically, the decay of a neutron also involves a neutrino. 1 0n neutron 1 1 H + 0 e -1 proton electron + 0 n 0 antineutrino Beta decay Actually, an anti-neutrino. The word “neutrino” comes from Enrico Fermi, meaning “little neutral one” in Italian. 1 0n neutron 1 1 H + 0 e -1 proton electron + 0 n 0 antineutrino Beta decay A neutrino is a particle with no charge and almost no mass. 1 0n neutron 1 1 H + 0 e -1 proton electron + 0 n 0 antineutrino Beta decay A neutrino carries off some of the energy in the decay of the neutron. 1 0n neutron 1 1 H + 0 e -1 proton electron + 0 n 0 antineutrino Beta decay When predicting the products of beta decay we will ignore neutrinos. 1 0n neutron 1 1 H + 0 e -1 proton electron + 0 n 0 antineutrino Start with a Li atom with 3 protons and 4 neutrons. Now there are 4 protons and 3 neutrons. Beta decay Suddenly a neutron decays! A beta particle goes zipping out of the nucleus. Beta decay A neutron decays to make a proton. The number of neutrons decreases by 1 The number of protons increases by 1 The mass number stays the same. The atomic number increases by 1 Beta decay decay product 6 14 C 7 14 N 0 e + -1 beta particle Beta decay The mass number stays the same. 14 C 6 14 N 7 The atomic number increases by 1. + -1 0 e Beta decay These add up to 14 6 14 C 7 14 N 0 e + -1 Notice that these add up to 6 Beta decay Zn-69 decays by beta emission. What is the decay product? 30 69 Zn -1 0 e + 69 ??? Ga 31 Beta decay Write the beta decay equations for: 1. 214 Pb 82 -1 2. 62 27Co 0 e -1 3. 113 ??? Ag 47 0 e 0 e -1 214 Bi + 83 + 62 Ni 28 + 48 113 Cd Review: decay equations Alpha: Go down two on periodic table Atomic number decreases by 2 Mass number decreases by 4 Beta: Go up one on periodic table Atomic number increases by 1 Mass number stays the same Nuclear energy All nuclear decay is accompanied by a release of energy. Alpha and beta particles have high kinetic energies. Gamma rays are electromagnetic energy. All have enough energy to ionize atoms. Nuclear energy An ion is a “charged atom” or group of atoms. Ionization occurs when electrons are removed from atoms by a, b or g radiation. This can result in damage to your body. cancer Nuclear energy Forms of ionizing radiation are: Alpha Beta Gamma X-rays Cosmic rays Neutrons Positrons Ultraviolet light (UV) can cause cancer, but it is not ionizing radiation.