* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Electric Field
Survey
Document related concepts
Electron mobility wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Elementary particle wikipedia , lookup
Anti-gravity wikipedia , lookup
Weightlessness wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Magnetic monopole wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Speed of gravity wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Fundamental interaction wikipedia , lookup
Field (physics) wikipedia , lookup
Maxwell's equations wikipedia , lookup
Electromagnetism wikipedia , lookup
Atomic nucleus wikipedia , lookup
Lorentz force wikipedia , lookup
Transcript
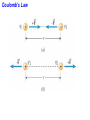
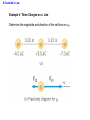
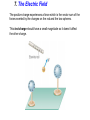
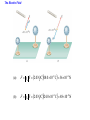
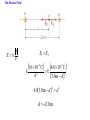
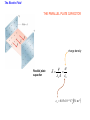
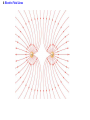
Unit 6 Electric Forces and Electric Fields · Electric Charge · Conduction and Induction · Electric Force (Coulomb's Law) • Electric field, shielding 1 The Origin of Electricity The electrical nature of matter is inherent in atomic structure. mp 1.673 1027 kg mn 1.675 10 27 kg me 9.1110 31 kg e 1.60 10 19 C Coulombs—unit for electric charge Electric Charge It has been known since ancient times that when certain materials are rubbed together, they develop an attraction for each other. (This can be seen today when you take clothes out of a dryer) In ancient Greece - people noticed that when thread was spun over a spindle of amber, the thread was attracted to the spindle. The Greek word for amber was "elektron," hence this force was called electric. Electric Charge In the 18th century, American Ben Franklin noticedw hen a rubber rod is rubbed by animal fur, the rod acquires a negative charge, and the animal fur acquires a positive charge. When a glass rod is rubbed by silk, the rod acquires a positive charge and the silk obtains a negative charge. Thus, two rubber rods after being charged would repel each other, while a rubber rod would be attracted to a glass rod. No new charge is created - instead, it is just separated - the positive charge acquired by one object is exactly equal in magnitude and opposite in sign to the charge lost by the other object. 1 The Origin of Electricity In nature, atoms are normally found with equal numbers of protons and electrons, so they are electrically neutral. By adding or removing electrons from matter it will acquire a net electric charge with magnitude equal to e times the number of electrons added or removed, N. q Ne 1 The Origin of Electricity Example 1 A Lot of Electrons How many electrons are there in one coulomb of negative charge? q Ne q 1.00 C 18 N 6.25 10 -19 e 1.60 10 C The Nature of Charge Like energy and momentum, charge is neither created nor destroyed, it is conserved. Opposite charges attract and like charges repel.A s a result negatively charged electrons are attracted to the positive nucleus. Despite the great mass difference, the charge on an electron is exactly equal in magnitude to the charge on a proton, and its magnitude is denoted by "e.“ An electron is said to have a charge of -e and a proton a charge of +e. Measurement of Charge The electron was discovered by J.J. Thomson in 1897, and in a series of experiments between 1909 and 1913, Robert Millikan and his graduate student, Harvey Fletcher, established the value of the charge, "e," on an electron. 2. Charged Objects and the Electric Force It is possible to transfer electric charge from one object to another. The body that loses electrons has an excess of positive charge, while the body that gains electrons has an excess of negative charge. 2 Charged Objects and the Electric Force LAW OF CONSERVATION OF ELECTRIC CHARGE During any process, the net electric charge of an isolated system remains constant (is conserved). 2 Charged Objects and the Electric Force Like charges repel and unlike charges attract each other. 2 Charged Objects and the Electric Force 1. An atom in its normal (non-ionic) state has no charge. This is due to the fact that atoms: A have only neutrons. B have no protons or electrons. C have equal numbers of protons and electrons. D have an equal number of protons and neutrons. 2. What object moves freely within the entire atom? A Electron. B Neutron. C Proton. D Nucleus. 3. An atom is composed of: A a central nucleus that is surrounded by neutrons. B an even distribution of electrons and protons in a spherical shape. C a central nucleus surrounded by electrons. D a central nucleus containing protons and electrons. 3. Conductors and Insulators Not only can electric charge exist on an object, but it can also move through an object. Substances that readily conduct electric charge are called electrical conductors. Materials that conduct electric charge poorly are called electrical insulators. Solids Solids are a form of matter whose nuclei form a fixed structure. Nuclei, and their protons and neutrons, are "locked" into position. Solids are classified as either conductors, insulators or semiconductors. In conductors, some electrons are free to move through the solid and are not bound to any specific atom. In insulators, electrons are bound to their atoms, and may move short distances, but much less than the electrons in a conductor. Semiconductors, depending on their situation, act as either conductors or insulators. 4. Charging by Contact and by Induction Charging by contact. 4. Charging by Contact and by Induction Charging by conduction involves conductors that are insulated from the ground, touching and transferring the charge between them. The insulator is necessary to prevent electrons from leaving or entering the spheres from Earth. Total Charge = -4Q (identical spheres very far apart) Question1: If a conductor carrying a net charge of 8Q is brought into contact with an identical conductor with no net charge, what will be the charge on each conductor after they are separated? Question2: Metal sphere A has a charge of -2Q and an identical metal sphere B has a charge of -4Q. If they are brought into contact with each other and then separated, what is the final charge on sphere B? 4 Charging by Contact and by Induction Charging by induction. 4 Charging by Contact and by Induction The negatively charged rod induces a slight positive surface charge on the plastic. Conduction Summary Through physical contact, a charged object will transfer a portion of its charge to a neutral object. Because of the Conservation of Charge, the amount of charge on the initially charged object will decrease. For example, a positively charged object will transfer positive charge to a neutral object, leaving it with a net positive charge. The amount of positive charge on the initial object will decrease. Similarly, a negatively charged object will transfer negative charge to a neutral object. Induction Summary A charged object will be brought close to a neutral object, but it will not touch it. The neutral object will be grounded - it will have an electrical conducting path to ground. The charged object will repel similar charges on the neutral object to the ground. Thus, the neutral object will be left with a charge opposite to the initially charged object. The initial object will not lose any charge the extra charge comes from the ground. As long as the ground is disconnected before the initial object is removed, the neutral object will gain charge. If the ground were left in place, once the initially charged object was removed, the neutral object will pass its gained charge back to the ground. 1. Sphere A carries a net positive charge, and sphere B is neutral. They are placed near each other on an insulated table. Sphere B is briefly touched with a wire that is grounded. Which statement is correct? A Sphere B remains neutral. B Sphere B is now positively charged. C Sphere B is now negatively charged. D The charge on sphere B cannot be determined without additional information. 2. If a positively charged rod touches a neutral conducting sphere and is removed, what charge remains on the sphere? What happens to the magnitude of the charge on the rod? A The sphere becomes positive and the rod's net charge stays the same. B The sphere becomes positive and the rod's net charge decreases. C The sphere becomes negative and the rod's net charge stays the same. D The sphere remains neutral and the rod's net charge stays the same. Demo Static electric charge: https://www.youtube.com/watch?v=QxZ6AWLpnUw Van de Graaf generator experiment: https://www.youtube.com/watch?v=ubZuSZYVBng 5. The Electroscope The electroscope measures electrical charge (both sign and magnitude). The conductor rod is insulated from the glass container. When the scope is neutral, the leaves hang down to due to their own mass. Electroscopes can be charged by conduction or induction. A neutral electroscope will become negatively charged when touched by a negatively charged object. Negative electrical charge will distribute across the electroscope and the gold leaves will repel, since they have the same charge, and like charges repel. The bar is moved away and there is now a negative net charge on the scope. Since negative charge moved from the rod to the electroscope, the rod now has less negative charge (Conservation of Charge). 1. When a negatively charged rod touches the top of a neutral electroscope, the gold leaves separate. What is the charge on the leaves? A Negative B Positive C Neutral 2. What is the source of the charge that is moved to the gold leaves? A The charged bar. B The ground. C The glass surrounding the leaves. 6. Coulomb’s Law Electrical Force: In the late 18th Century, several physicists (Joseph Priestly and John Robison) reasoned (and Robison measured) that the force between two objects followed the same principles as the gravitational force and that the force between two charged objects depends on the inverse square of the distance between them Charles Coulomb published a paper (1785), based on detailed experiments, that definitively proved the above, and that the force was also proportional to the size of the charges. He used a torsion balance which was based on the same principle as Henry Cavendish's experiment that measured the gravitational constant. Coulomb’s Law 6 Coulomb’s Law COULOMB’S LAW The magnitude of the electrostatic force exerted by one point charge on another point charge is directly proportional to the magnitude of the charges and inversely proportional to the square of the distance between them. F k q1 q2 8.85 10 12 C 2 N m 2 r2 k 1 4o 8.99 109 N m 2 C 2 Coulomb's Law is used to calculate the magnitude of the force. Each object exerts the same force on the other - except in opposite directions (Newton's third law applies to all forces, not just mechanical ones). Since electrical force, like all forces, is a vector, you need to specify the direction of the force magnitude determined by Coulomb's Law. This is done by looking at the sign of both charges (like charges repel & opposite charges attract). 6 Coulomb’s Law Example 3 A Model of the Hydrogen Atom In the Bohr model of the hydrogen atom, the electron is in orbit about the nuclear proton at a radius of 5.29x10-11m. Determine the speed of the electron, assuming the orbit to be circular. F k q1 q2 r2 6 Coulomb’s Law F k q1 q2 r2 8.99 10 9 N m C 1.60 10 2 2 5.29 10 11 m 19 C 2 2 8.22 10 8 N F mac mv2 r v Fr m 8.22 10 N5.29 10 8 9.1110 kg -31 11 m 2.18 10 6 ms Electrical Force relationship to Gravitational Force Both forces are expressed using a similar mathematical formula, where the magnitude of the force decreases as 1/r 2. Electrical force can be attractive or repulsive (like charges repel, opposite charges attract). Gravitational force is always attractive. The electrical force is on the order of 1036 times stronger than the gravitational force! 2. A +20.0 μC point charge is located 20.0 cm away from a -40.0 μC point charge. What is the force on each due to the other? 3. What is the distance between two charges +7.8 μC and +9.2 μC if they exert a force of 4.5 mN on each other? 4. A -4.2 μC charge exerts an attractive force of 1.8 mN on a second charge which is a distance of 2.4 m away. What is the magnitude and sign of the second charge? 5. Two equal negatively charged objects repel each other with a force of 18 mN. What is the charge on each object if the distance between them is 9 cm? How many extra electrons are on each object? 6 Coulomb’s Law Example 4 Three Charges on a Line Determine the magnitude and direction of the net force on q1. 6 Coulomb’s Law F12 k F13 k q1 q2 r 2 q1 q3 r 2 8.99 10 9 8.99 10 N m 2 C2 3.0 106 C 4.0 106 C 0.20m2 9 N m 2 C2 3.0 106 C 7.0 106 C 0.15m2 F F12 F13 2.7 N 8.4 N 5.7N 2.7 N 8.4N Superimposition of Electrical Forces Follow this procedure: 1. Assume all charges, other than the one that the initial net force is being calculated for, are immobile - this will allow the determination of the direction of the individual initial forces. 2. Draw a free body diagram for each charge, using the fact that opposite charges attract and like charges repel. 3. Use Coulomb's Law to find the magnitude of each force. 4. Sum the forces, taking into account that they are vectors with direction and magnitudes. Use the free body diagrams to assign signs to the forces - if they point to the right, they are positive; if they point to the left, they are negative. 7. The Electric Field The positive charge experiences a force which is the vector sum of the forces exerted by the charges on the rod and the two spheres. This test charge should have a small magnitude so it doesn’t affect the other charge. The Electric Field Example 6 A Test Charge The positive test charge has a magnitude of 3.0x10-8C and experiences a force of 6.0x10-8N. (a) Find the force per coulomb that the test charge experiences. (b) Predict the force that a charge of +12x10-8C would experience if it replaced the test charge. (a) (b) F 6.0 10 8 N 2.0 N C 8 qo 3.0 10 C F 2.0 N C 12.0 108 C 24 108 N The Electric Field DEFINITION OF ELECRIC FIELD The electric field that exists at a point is the electrostatic force experienced by a small test charge placed at that point divided by the charge itself: F E qo SI Units of Electric Field: newton per coulomb (N/C) 7 The Electric Field It is the surrounding charges that create the electric field at a given point. The Electric Field Example 7 An Electric Field Leads to a Force The charges on the two metal spheres and the ebonite rod create an electric field at the spot indicated. The field has a magnitude of 2.0 N/C. Determine the force on the charges in (a) and (b) The Electric Field (a) F qo E 2.0 N C 18.0 108 C 36 108 N (b) F qo E 2.0 N C 24.0 108 C 48 108 N The Electric Field Electric fields from different sources add as vectors. The Electric Field Example 10 The Electric Field of a Point Charge The isolated point charge of q=+15μC is in a vacuum. The test charge is 0.20m to the right and has a charge qo=+15μC. Determine the electric field at point P. F E qo F k q1 q2 r2 The Electric Field F k q qo r2 8.99 10 E 9 N m 2 C 2 0.80 10 6 C 15 10 6 C 0.20m 2 F 2.7 N 3.4 106 N C -6 qo 0.80 10 C 2.7 N The Electric Field q qo 1 F E k 2 qo r qo The electric field does not depend on the test charge. Point charge q: Ek q r2 The Electric Field Example 11 The Electric Fields from Separate Charges May Cancel Two positive point charges, q1=+16μC and q2=+4.0μC are separated in a vacuum by a distance of 3.0m. Find the spot on the line between the charges where the net electric field is zero. Ek q r2 The Electric Field Ek q r2 E1 E 2 16 10 C 4.0 10 C k k 6 d2 6 3.0m d 2 4.03.0m d d 2 2 d 2.0 m The Electric Field THE PARALLEL PLATE CAPACITOR charge density Parallel plate capacitor E q o A o 8.85 10 12 C 2 N m 2 8. Electric Field Lines Electric field lines or lines of force provide a map of the electric field in the space surrounding electric charges. 8. Electric Field Lines Electric field lines are always directed away from positive charges and toward negative charges. 8 Electric Field Lines Electric field lines always begin on a positive charge and end on a negative charge and do not stop in midspace. 8 Electric Field Lines The number of lines leaving a positive charge or entering a negative charge is proportional to the magnitude of the charge. 8 Electric Field Lines 9 The Electric Field Inside a Conductor: Shielding At equilibrium under electrostatic conditions, any excess charge resides on the surface of a conductor. At equilibrium under electrostatic conditions, the electric field is zero at any point within a conducting material. The conductor shields any charge within it from electric fields created outside the conductor. 9 The Electric Field Inside a Conductor: Shielding The electric field just outside the surface of a conductor is perpendicular to the surface at equilibrium under electrostatic conditions. 9 The Electric Field Inside a Conductor: Shielding Conceptual Example 14 A Conductor in an Electric Field A charge is suspended at the center of a hollow, electrically neutral, spherical conductor. Show that this charge induces (a) a charge of –q on the interior surface and (b) a charge of +q on the exterior surface of the conductor.