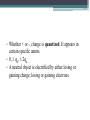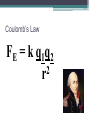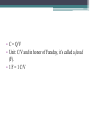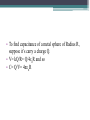* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electrostatics
Survey
Document related concepts
Potential energy wikipedia , lookup
Casimir effect wikipedia , lookup
Work (physics) wikipedia , lookup
Anti-gravity wikipedia , lookup
Magnetic monopole wikipedia , lookup
Electromagnetism wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Fundamental interaction wikipedia , lookup
Speed of gravity wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Maxwell's equations wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Field (physics) wikipedia , lookup
Lorentz force wikipedia , lookup
Transcript
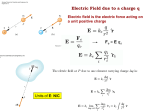
Electrostatics • Electostatics: the study of charges at rest. • Charges give rise to electric force (electromagnetic force, to be precise) • Like charges repel, opposites attract. • 2 types of charge: + and – • In the majority of cases, only the negative charges are mobile. • An object that contains the same amnt of + and – charges in close proximity attracts and repels an external charge equally and thereby cancels it own ability to exert a net force. It is neutral. • An e- is negatively charged. • There is no way to discharge an electron and make it neutral. If the electron is a fundamental particle, charge is an inseparable aspect of the thing itself. • We have experimentally determined the charge of an electron (qe). Electric charge comes in whole number multiples of that basic amnt. • Whether + or -, charge is quantized. It appears in certain specific amnts. • 0, ± qe, ± 2qe … • A neutral object is electrified by either losing or gaining charge; losing or gaining electrons. Conservation of Charge • The total charge (difference between the amnt of + and – charge) within an isolated system is always constant. Charging by friction • Ex: shuffling across the carpet in socks. • Outer electrons are the least strongly bound and most easily shed. • Different materials have different affinities for electrons. • When in contact, one material may give up some of its electrons to the other. • Depends on the materials. Triboelectric sequence • “tribo” friction • Materials towards the top of the list tend to lose electrons easily, those near the bottom tend to gain them effectively. • http://www.siliconfareast.com/tribo_series.htm Transfer of + charge • A positively charged object when placed near something neutral will draw e- away from the neutral object leaving both positively charged. • ONLY e- are transferred. But the system behaves as though positive charge flowed from the charged object to the neutral. Insulators and Conductors • A conductor allows charge introduced anywhere within it to flow freely and redistribute. (ex: metals) • Good conductors hold onto their outer e- weakly. • There is no perfect insulator, all allow some redistribution of charge. • Air is a good insulator (esp dry air). • If enough negative charge builds up on an object, electrons, under the force of mutual repulsion, may be emitted into the surrounding air. • This increases the amnt of free e- and ions in the air, temporarily causing the air to become an electrical conductor. • The temperature increase causes some of the atoms to emit light and sound (a spark). Conduction • A cluster of identical charges introduced on a conductor experiences a mutual repulsion that sends them scurrying until they can separate no farther and are as far away from each other as possible. • No matter what the shape of the conductor, excess charge always resides on its outer surface. • Another manifestation of the flow of e- in a conductor is the manner in which charge is transferred. • A negative conductor that touches an uncharged metal body. e- are propelled onto the neutral body by their mutual repulsion. • Flow like a liquid until charge is equalized on both objects. Equilibrium. • Any good conductor in good contact with the Earth (like water pipes in a building) will carry off all the charge one could want to discard, depositing I into the moist ground. • To ground Induction • Charge is induced when no physical contact between the objects. Electric Force • Mid 18th C. question of the nature of the force acting between charges was a major scientific issue. • Speculation was very Newtonian, relying on discoveries regarding gravity. • Bernoulli, with his crude experiments, also confirmed that electrical attraction/repulsion seemed to follow an inverse square law. • Ben Franklin was able to observe that there is no charge on the inside wall of a hollow electrified conductor. All charge was on the outside. • All this led up to Charles Augustin de Coulomb’s definitive study on electrical force (1785). Coulomb’s Law FE = k q1q2 2 r • The force acts between two charges is directly proportional to the product of the charges and inversely proportional to the square of the distance separating them. • + force means repulsive, - means attractive • Units for charge: coulomb (C) • k =8.98755179 x 109 Nm2/C2 • The charge of an e- is -1.06217733 x 10-19 C Example 1 A Hydrogen atom consists of an electron of mass 9.1094 x 10-31 kg, moving about a proton of mass 1.6276 x 10-27 kg at an avg distance of 0.53 x 10-10 m. Find the electric and gravitational forces acting between the two particles. • Experimentally confirmed that when several charges are present, each exerts a force given by Coulomb’s Law on every other charge. • The interaction btwn any two charges is independent of the presence of all other charges. • The net force on any one charge is the sum of all the forces exerted on it due to each of the other charges interacting with it independently. Example 2 Drawing on board shows three tiny uniformly charged spheres. Determine the net force acting on the middle sphere due to the other two. Example 3 Drawing on the board depicts three small charged spheres at the vertices of a 3-4-5 right triangle. Calculate the force (mag and dir) exerted on q3 by the other two charges. Electric Field • A field of force exists in a region of space when an appropriate object placed at any point therein experiences a force. • Michael Faraday was the first to introduce a visual representation of the electric force field. • Uses lines of force. • The force experienced by a + test charge at any point in space is in the direction tangent to the line offorce at that point. • Flow out of + and into – • The more lines drawn in a region (i.e., the denser the concentration of the lines), the stronger the field they represent. • The farther apart the lines, the weaker the field. • We define the electric field (E with an arrow on top) at a point in space to be the electric force experienced by a + test charge at that point divided by that charge. E = F/qo • Force on a point charge could be found with same eqn. F = Eq Example 4 • At a particular moment the electric field at a point 30 cm above an electric blanket is 250 N/C, straight up. Compute the force that acts on an e- at that location, at that moment. • Electric field of a point charge E = k (q/r2) Allows us to calculate the E-field anywhere in space due to a known distribution of point-charges (e-, p+, whatever). Each charge contributes a field to the overall E-field. Example 5 • Drawing on the board shows two point-charges (or tiny charged spheres) each of 10nC separated in air by a distance of 8.0 m. Compute the electric field at points A, B, and C. • English electrical engineer, Oliver Heaviside, pointed out around 1892 that most of the eqns for the E-fields of different charge distributions contained a factor of 4π. Suggested writing the k in Coulomb’s law to reflect the 1/4 π and a new constant ε, so that k= 1 4πε • ε is called the permittivity (from Latin permittere, to let go through). • In a vacuum, εo = 8.8541878 x 10-12 C2/Nm2 Relative permittivity is ε/ εo Also called dielectric constant (Ke) and is a unit-less ratio. Example 6 • A point charge of 10 micro C is surrounded by water with a dielectric constant of 80. Calculate the magnitude of the electric field 20 cm away. Field Lines • They diverge away from a + point charge and converge on a – point charge. • We call the former a source of the field, the latter the sink. • E field lines always begin on the positive charge and end on the negative charge. • Lines of the net field never cross • Dipole: positive and negative charges of the same magnitude. Gauss’s Law • Allows us to calculate the E field of any symmetrical charge distribution. • K. F. Gauss. German mathematician and astronomer (19th C.) • The product of the area and the strength of the field passing perpendicularly through it is the flux of the field. • Think of it like Continuity Eqn in fluids. • A1 Eperp1 = A2 Eperp2 • For any closed surface the net flux of the E field passing through that surface, whatever its shape, is proportional to the total enclosed charge. • Gauss’s Law becomes: check board. • Can’t use calculus, so we only deal in symmetrical charge distributions. • Charge density • Linear charge density: λ = Q/L Surface charge density: σ = Q/A Volume charge density: ρ = Q/V Example 7 • A long, straight wire of length L, in air, carries a uniform positive charge Q. Use Gauss’s Law to find the electric field at a perpendicular distance r from the wire at a point that is far from the conductor’s ends. To operate in a field region free of the complications of end effects, we limit the analysis to the domain where L >> r. • When a charge is made to move against the influence of an electric field, it will experience a change in it’s electrical U. • Fig 16 • The opposite happens when we allow it to move w/ the field. As speed increases, UE decreases Electric Potential • Aka Potential, voltage • Potential energy per unit charge at any point in space you want to find. • Units: J/C • Volts (V), after Alessandro Volta Potential = (UE)/q Example 1 • The most commonly used unit of E field is the volt per meter. Show that this is consistent with the units introduced thus far. • ΔV (potential difference) = Vf – Vi • ΔV = W/q Example 2 • A small sphere carrying a + charge of 10 microCoulombs is moved against an E field through a potential difference of 12.0 V. How much work was done by the applied force in raising the potential of the sphere? • There are no cosmic signposts floating around saying “HERE BE 0 ELECTRIC U.” • (sign posts talk pirate style) • Only differences in potential are important. • Work done against the E field increases U, work done by the E field decreases U • Work done on a charge in moving it from point A to B is independent of the path taken • When a particle with charge +qe moves in a field, dropping 1 V in potential, it will decrease its electrical U by ΔU= qe ΔV = 1.6 x 10-19 J. • A lot of stuff on the atomic level involve energies of about this amount, so we measure this bit of energy with a new unit: electron volt (eV). • 1 eV = 1.6 x 10-19 J Example 3 • Figure 16 shows the basic elements of a CRT. Electrons are “boiled” off a heated cathode and emerge through a pinhole, being drawn toward the first anode, which is at a relatively small positive potential above the cathode. A second anode that is 20 kV above the cathode accelerates the beam up to speed. Determine the change in U of each e- . Assume that each e- has negligible motion at the cathode. Find its final speed. • In a uniform E field, VB – VA = ±𝐸𝑑 • V is + (voltage rise) when the displacement is opposite to the field and – when it’s same dir’n. • In a 12 V battery, the + terminal is 12 V higher than the negative terminal. Example 4 • If the separation of the plates in Fig 16.7 is 2.00 mm, determine the magnitude of the E field in the air gap. • VB – VA = kQ [(1/rB) – (1/rA)] • V = (kQ)/r • This is the potential at a distance r from a positive point-charge Q measured with respect to the zero at infiniti. Example 4 • What is the potential difference encountered in going from close in (point B) to far out (point A) in Fig 16.8, if the tiny sphere carries a charge of 10 microC, rA = 20 cm and rB = 10 cm? Conservation of Charge • Charge is conserved. • Simplest way to look at it: if charge disappears, then you have problems with conservation of energy Capacitors • Volta coined the term “electrical capacity.” Only in Italian, I’m assuming. • At a give potential (V), the amnt of charge (Q) that can be stored by a body depends on its physical characteristics, all of which we lump together under the name capacitance (C) • C = Q/V • Unit: C/V and in honor of Faraday, it’s called a farad (F). • 1 F = 1 C/V • To find capacitance of a metal sphere of Radius R, suppose it’s carry a charge Q. • V= kQ/R= Q/4εoR and so • C= Q/V= 4πεoR Example 7 • Determine the capacitance of an isolated metal sphere 50 cm in diameter and immersed in a vacuum. Parallel Plate Capacitor • Ben Franklin among the first. • Sandwiched insulating glass between metal plates. • Introducing the second, negatively charged, plate drops the potential of the first plate, which can be restored to its original value by further increasing the charge. • The second plate considerably increase the ability of the parallel plate capacitor to store charge at a given voltage. • ΔV = Ed • E = σ/ε= Q/A ε Where A is the area of each plate and σ = Q/A ΔV = Ed= Q/(A ε) C=Q/ ΔV= Q/(Qd/A ε) So… C = εA/d