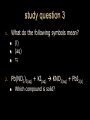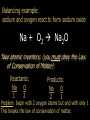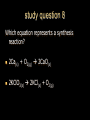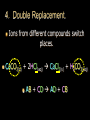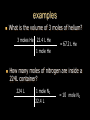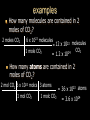* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Mole
Process chemistry wikipedia , lookup
Sodium hydroxide wikipedia , lookup
Electron configuration wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Click chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Electrolysis of water wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Biochemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Sodium hypochlorite wikipedia , lookup
Hydrogen atom wikipedia , lookup
Transition state theory wikipedia , lookup
Electrochemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
History of chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Molecular dynamics wikipedia , lookup
Chemical bond wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
History of molecular theory wikipedia , lookup
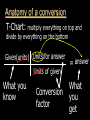
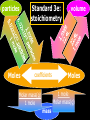
Stoichiometry. Chapter 9, 10, & 11 Standard 3 Ms. Siddall vocabulary 1. 2. 3. 4. 5. 6. 7. 8. 9. Compound Chemical formula Coefficient Stoichiometry Mole Molar mass Molar volume Avogadro’s number Standard temperature and pressure Anatomy of a chemical formula chemical formula: a combination of symbols and numbers that describe the amount and type of atoms that form a compound. Example: CuSO4(aq) Atomic symbols describe the type of atoms in the compound (copper, sulfur, oxygen) subscript numbers describe the number of atoms in the compound (1 copper, 1 sulfur, 4 oxygen) physical state of the compound is described using subscript letters (aq = aqueous) study question 1 Na2O(s) In the above formula: 1. How many sodium atoms? 2. How many oxygen atoms? 3. What is the physical state? 4. Is the compound ionic or covalent? Standard 3a: describing chemical reactions An equation describes a chemical reaction or a physical change Reactants: chemicals that react Products: chemicals that are formed e.x. sodium + oxygen sodium oxide Na(s) + O2(g) Na2O(s) reactants product study question 2 Pb(NO3)3(aq) + KI(aq) KNO3(aq) + PbI3(s) 1. 2. 3. Label the reactants and the products in the reaction above Are the reactants and products covalent or ionic? How many oxygen atoms are in the compound Pb(NO3)3? Symbols describing chemical reactions Copy table 11.1 (page 323) That’s right – open your book to page 323 and copy that table into your notes… go on… do it! This spot had better not be blank when I check your notebook! study question 3 1. What do the following symbols mean? 2. (l) (aq) Pb(NO3)3(aq) + KI(aq) KNO3(aq) + PbI3(s) Which compound is solid? Balancing chemical equations The Law of Conservation of Matter: Matter cannot be created or destroyed. For chemical equations: The total number of each type of atom must be the same before and after the reaction Law: Thou shall not create or destroy matter example Sodium reacts with oxygen to produce sodium oxide Na(s) + O2(g) Na2O(s) How many sodium atoms react? 1 How many sodium atoms are in the product? 2 This violates the law of conservation of matter! study question 4 NaI(s) + Cl2(g) NaCl(s) + I2(s) Count the number of Count the number of atoms on the reactants atoms on the products side side Sodium ____ Sodium ____ Iodine ____ Iodine ____ Chlorine ____ Chlorine ____ Does this equation obey the law of conservation of matter? Example: 4 Na(s) + O2(g) 2 Na2O(s) SUBSCRIPTS CAN NOT CHANGE! Coefficients are used to balance the equation number of atoms or formulas needed in the reaction. These apply to the entire formula (all the atoms) 4Na = 4 sodium atoms 2Na2O = 4 sodium atoms and 2 oxygen atoms study question 5 2Cu(s) + O2(g) 2CuO(s) Count the number of Count the number of atoms on the reactants atoms on the products side side copper ____ copper ____ Oxygen ____ Oxygen ____ Does this equation obey the law of conservation of matter? Rules of Balancing Equations 1. 2. Write the equation using correct formulas. You may NOT change the formula in any way. Balance the equation using coefficients Balancing example: sodium and oxygen react to form sodium oxide Na + O2 Na2O Take atomic inventory: (you must obey the Law of Conservation of Matter) Reactants: Na O 1 2 Products: Na O 2 1 Problem: begin with 2 oxygen atoms but end with only 1 This breaks the law of conservation of matter. Na + O2 2Na2O (= Na2O + Na2O) solution: Add the coefficient ‘2’ in front of Na2O Take atomic inventory again: Reactants: Na O 1 2 Products: Na O 4 2 Problem: begin with 1 sodium atom but end with 4. This breaks the law of conservation of matter. 4 Na + O2 2Na2O solution: Add the coefficient ‘4’ in front of Na Take atomic inventory again: Reactants: Na O 4 2 Products: Na O 4 2 4 sodium atoms combine with 1 oxygen molecule to form 2 formula units of sodium oxide. This equation obeys the Law of Conservation of Matter. study question 6 BALANCE THE FOLLOWING REACTION: H2(g) + O2(g) H2O(l) Balancing with polyatomic ions Sometimes polyatomic ions break apart in a chemical reaction and sometimes they do not e.x. sulfate appears on both sides of the reaction so SO4 can be treated like one atom: Mg(s) + CuSO4(aq) MgSO4(aq) + Cu(s) e.x. carbonate breaks apart so atoms must be balanced individually: CaCO3(aq) + HCl(aq) CaCl2(aq) + H2O(l) + CO2(g) study question 7 Balance the following equations: 1. Na2CO3(s) + HCl(aq) NaCl(aq) + CO2(g) + H2O(l) 2. K2SO4(aq) + CaCl2(aq) CaSO4(s) + KCl(l) Types of Chemical Reactions. 1. Combination. Also called synthesis Two or more reactants combine to form one product e.x. 2 Na(s) + Cl2(g) 2 NaCl(s) A + B AB study question 8 Which equation represents a synthesis reaction? 2Ca(s) + O2(g) 2CaO(s) 2KClO3(s) 2KCl(s) + O2(g) 2. Decomposition. One reactant decomposes to form two or more products. 2H2O(l) 2H2(g) + O2(g) AB A + B study question 9 Which equation represents a decomposition reaction? Ca(s) + O2(g) CaO(s) 2KClO3(s) 2KCl(s) + O2(g) 3. Single Replacement. An atom replaces an ion in a compound. Mg(s) + CuSO4(aq) MgSO4(aq) + Cu(s) Cl2(g) + 2KI(aq) I2(s) + 2KCl(aq) A + BC AC + B study question 10 Which equation represents a single replacement reaction? • 2NaI(s) + Cl2(g) 2NaCl(s) + I2(s) • 2NaI(aq) + Pb(NO3)2(aq) 2NaNO3(aq) + PbI2(s) 4. Double Replacement. Ions from different compounds switch places. CaCO3(s) + 2HCl(aq) CaCl2(s) + H2CO3(aq) AB + CD AD + CB study question 11 Which equation represents a double replacement reaction? • 2NaI(s) + Cl2(g) 2NaCl(s) + I2(s) • 2NaI(aq) + Pb(NO3)2(aq) 2NaNO3(aq) + PbI2(s) 5. Combustion reactions. A compound reacts with oxygen often produces CO2 & H2O e.x. C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) Note: a combustion reaction can also be a decomposition or a combination reaction study question 12 Write the balanced equation for the reaction of CO with O2 to form CO2 and identify the type of reaction. Standard 3e: The Arithmetic of Equations. A balanced equation shows the amount of each reactant and product needed or produced in any reaction. Example. Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g) One atom of magnesium combines with 2 formula units of HCl to form one formula unit of magnesium chloride and one molecule of hydrogen gas. Use equation coefficients to solve quantitative problems. study question 13 4Na(s) + O2(g) 2Na2O(s) 1. 2. 3. How many molecules of oxygen are needed to react with 4 atoms of sodium? How many oxygen atoms is that? How many formula units of sodium oxide are produced when 4 atoms of sodium are used? Example. Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g) If 1 atom of magnesium is used, 2 formula units of HCl are needed to react. If 6 atoms of magnesium are used, how many formula units of HCl are needed? 6 atom Mg 2 Fo.U. HCl 1 atom Mg = 12 Fo.U. HCl Anatomy of a conversion T-Chart: multiply everything on top and divide by everything on the bottom Given(units) Units for answer Units of given What you know Conversion factor = answer What you get study question 14 Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g) if 4 molecules of H2 are created 1. 2. how many Fo.U. of HCl are needed? How many atoms of Mg are used? Standard 3b: The Mole Atoms and molecules are so small scientists must use a large number of atoms, molecules or formula units in order to observe chemical reactions. This large number is called: A Mole Standard 3c: Avogadro’s Number The number of particles in one mole = Avogadro’s number = 6.02 x 1023 = 602,000,000,000,000,000,000,000 Particles = atoms, molecules, formula units, donuts… study question 15 How many dollars would you have if you had Avogadro’s number of dollars? Avogadro’s number is convenient because a mole of any chemical is easily measured in the laboratory. Instead of using 6.02 x 1023 atoms we use 1 mole of atoms. study question 16 4Na(s) + O2(g) 2Na2O(s) 1. 2. 3. How many moles of O2 are needed to react with 4 moles of sodium? How many moles of O2 are needed to react with 2 moles of sodium? How many moles of Na2O are produced when 2 moles of sodium are used? particles Moles Standard 3e: stoichiometry coefficients volume Moles 1 mole Molar mass(g) Molar mass(g) 1 mole mass 1 Mole of any atom has a mass (in grams) numerically equivalent to the mass of a single atom (in amu). This mass is displayed on the periodic table. study question 17 What is the mass of one mole of: Aluminum? Carbon? Standard 3d: Molar Mass = the mass of one mole of any substance. Example: Na2O Sodium = 23g/mole Oxygen = 16g/mole 1 mole Na2O: (2x23g/mole) + 16g/mole = The molar mass for Na2O = 62g/mole study question 18 calculate the molar mass of AlCl3 (don’t forget units!) examples What is the mass of 3 moles of sodium hydroxide? Molar mass! 3 moles NaOH 40 g NaOH 1 mole NaOH = 120 g NaOH How many moles of carbon dioxide are in a sample weighing 88g? 88g CO2 1 mole CO2 44g CO2 = 2 moles CO2 study question 19 A person produces just less than 0.5 moles CH4 per day. How many grams is that? The volume of a Mole 1 mole of any GAS has a volume of 22.4L at standard temperature and pressure Standard temperature = 0°C Standard pressure = 1atm study question 20 1. What is the volume of 2 moles of gas? 2. What is the volume of 0.5 moles of gas? (At standard temperature and pressure) examples What is the volume of 3 moles of helium? 3 moles He 22.4 L He 1 mole He = 67.2 L He How many moles of nitrogen are inside a 224L container? 224 L 1 mole N2 22.4 L = 10 mole N2 study question 21 A person produces just less than 11.2L CH4 per day. How many moles is that (assume STP conditions)? Example. Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g) If 73g hydrochloric acid are used, how many liters of hydrogen gas are produced? 73 g HCl 1 mole HCl 36.5 g HCl 1 mole H2 22.4L H2 2 moles HCl 1 mole H2 = 22.4 L H2 study question 22 3H2(g) + N2(g) 2NH3(g) If one mole of nitrogen is used how many moles of hydrogen are needed? How many liters of hydrogen are needed to produce 17g of NH3(g)? examples How many molecules are contained in 2 moles of CO2? 2 moles CO2 6 x 1023 molecules = 12 x 1023 molecules CO2 1 mole CO2 24 = 1.2 x 10 How many atoms are contained in 2 moles of CO2? 2 mol CO2 6 x 1023 molcs 3 atoms 1 mol CO2 1 molc CO2 = 36 x 1023 atoms = 3.6 x 1024 study question 23 A cow produces about 1500L CH4 per day. How many molecules is that?