* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Notes Unit 5-4
Survey
Document related concepts
Atomic nucleus wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Process chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Chemical bond wikipedia , lookup
Isotopic labeling wikipedia , lookup
Metalloprotein wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
History of molecular theory wikipedia , lookup
Transcript
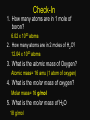
Warm up 11-13-15 1. Balance and List the type of reaction a) _ Al + _ Pb(NO3)2 ---> _ Pb + _ Al(NO3)3 b) _ C3H8 + _ O2 ---> _ CO2 + _ H2O 2. A reaction yield 25%, how many grams of CO2 produced if the expected amount is 80g? Agenda Homework Go over homework Notes Unit 5-4 Mole Poster Due Monday Nov 20 - Quiz Unit 5 Nov 21 - Online HW Unit 5 Dec 9 - Deadline to retake quizzes and turn in absent work 1. Balance and List the type of reaction a) _ Al + _ Pb(NO3)2 ---> _ Pb + _ Al(NO3)3 b) _ C3H8 + _ O2 ---> _ CO2 + _ H2O 2. A reaction yield 25%, how many grams of CO2 produced if the expected amount is 80g? Unit 5-4 Notes Stoichiometry & Moles STOICHIOMETRY • The quantitative aspects of chemical reactions. • Precise measurement, a recipe for chemical reactions How do we count? • 1 “dozen” donuts = ____ donuts • 1 “dozen” eggs = _____ eggs • 1 “foot” = ______ inches A “Mole” • 1 mole = 6.02 x 1023 particles • Mole = amount of a substance “mol” • Avogadro’s Number • Based on the weight of carbon-12 atoms. 1776 – 1856 Just How Big is a Mole? • Enough soft drink cans to cover the surface of the earth to a depth of over 200 miles. • 602,200,000,000,000,000,000,000 unpopped popcorn kernels and spread them across the United States of America, the country would be covered in popcorn to a depth of over 9 miles. • If we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count the atoms in one mole. Molar Mass • The mass of 1 mole (not 1 atom) of a substance • Units: g/mol • Molar Mass Copper 6.02 x 1023 atoms 63.5 g/mol vs. Atomic Mass Copper 1 atom 63.5 amu Molar Mass • How to find the mass of compounds • What is the molar mass of sugar? • Sugar = C6H12O6 Check-In 1. How many atoms are in 1 mole of boron? 6.02 x 1023 atoms 2. How many atoms are in 2 moles of H2O? 12.04 x 1023 atoms 3. What is the atomic mass of Oxygen? Atomic mass= 16 amu (1 atom of oxygen) 4. What is the molar mass of oxygen? Molar mass= 16 g/mol 5. What is the molar mass of H2O 18 g/mol Mole Project (10 points) Individual Project Due Next Class -Create a “mole” theme poster -Hand drawn and Color -Include a 3 key terms from today’s note. Example theme: Moleo and Juliet Molethello Han Molo Moledamort Moleius Caesar Whack-a-moley Angry Mole Pepto Bismole Pikamole InstaMole