* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Virus-Induced Immunopathology
Survey
Document related concepts
Influenza A virus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Orthohantavirus wikipedia , lookup
Ebola virus disease wikipedia , lookup
West Nile fever wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Hepatitis C wikipedia , lookup
Marburg virus disease wikipedia , lookup
Henipavirus wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Transcript
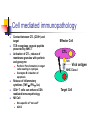
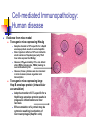
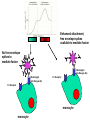
Virus-Induced Immunopathology Cell mediated Immunopathology Antibody-mediated Immunopathology Virus-initiated Immunopathology Cell mediated immunopathology Contact between CTL (CD8+) and target TCR recognizes cognate peptide presented by MHC I Activation of CTL; release of membrane granules with perforin and granzyme Perforin: Pore formation in target cells resuting in cytolysis Granzyme B: induction of apoptosis Release of Inflammatory cytokines (TNF-a, IFN-g, ILs) CD4+ T cells can enhance CD8mediated immunopathology NK Cell Non-specific of “non-self” ADCC Effector Cell CTL TCR Viral antigen MHC Class I Target Cell Cell-mediated Immunpathology: LCMV, an experimental model Inflammatory Cytokines Cytopathic attack on choriodal cells can damage meninges Convulsions (?) Effector Cell Nitric Oxide CTL TCR Viral antigen MHC Class I NOS Inflammatory Macrophage Infected Choriodal Cells Cell-mediated Immunpathology: LCMV, an experimental model Lymphocytic Choriomeningitis Virus causes fatal choriomeningitis (inflammation of meninges) in immunocompetent mice, but not in immunosuppressed mice Intracerebral Innoculation e.g. via Cyclophosphamide Rx, or irradiation Adoptive Transfer: Syngeneic normal mice “immunized” IP with LCMV. Anti-serum or lymphocytes then adoptively transferred Log10 Virus Titer Serum GLDH A: Perforin is required for CTL effector function B: Perforin, hence CTL function, is required for virus clearance (IV challenge) C: Perforin, CTL activity, mediates fatal choriomeningitis (IC infection) D: CTL is also involved in hepatopathology (liver damage) by hepatotropic LCMV Percent Alive % Cr51 release CTL activity is required for LCMV immunopathology Immune mediated disease: Tissue destruction in addition to inflammation Hepatotropic LCMV Liver destruction Cerebellar involution in newborn rats Pathogenic consequences: severe ataxia Immunosuppressed Non-Immunosuppressed CD4+ T cells can enhance immune pathology (N.B. in the book, should be RSV should be Respiratory Syncytial Virus, NOT Rous Sarcoma Virus) Respiratory Syncytial Virus: virus induced immunopathology (bronchioaveolitis) is more dependent on CD4 T lymphocytes Immu nization (day 0) Forma lininactivated RSV (all gro ups) Depletion days 18, 20, 23 None CD8 CD4 CD8 + CD4 Intranasal RSV infection day 21 YES YES YES YES Alveolitis (% lung area) day 25 9.6% 3.9% 0.5% 0.3% Complement Fixation (neg.) IgG3>IgG1>IgG3>>>(IgG4) Depletion is usually accomplished by infusing mice with antibodies (complement-fixing) against the specific cellsurface molecule Passsive transfer of antibodies (from RSV infected animals) cannot re-produce aveolitis Conclusion: alveolitis is mediated mainly by CD4+ effector cells Cell-mediated Immunopathology: Human disease Clinical disease associated with development of hightiter antibodies Presence of high titer virus (viremia) in the absence of clinical disease (hepatitis) suggest that the disease is not caused by infection per se Anti-HBsAg may contribute to transient acute hepatitis, but may synergize with CTL mediated clerance of virus from hepatocytes CTL response itself can result in acute hepatitis % Maximum Hepatitis B Virus infection % Maximum HBV All three particles contain HBsAg (also known as the major protein) The L(large) and M(middle) are also contained in the Dane particles The L protein when expressed alone gets trapped in ER ~45 nm “Dane”particles (infectious) ~20 nM spheres HBsAg (major) and middle proteins and host lipids; No nucleic acid, non-infectious Present in 103 to 106 fold excess over Dane particles Can reach 1012 particles/ml in infected patients Highly immunogenic, original source of 1st generation HBV vaccine ~20 nM in diameter filaments (contains the L protein, but lack nucleic acids; Non-infectious) Cell-mediated Immunopathology: Human disease Evidence from mice model Transgenic mice can be made to express large, middle or major envelope proteins Wt. Mice can be vaccinated with envelope proteins and Ag-specific CTL clones can be made directed against specific epitopes in large, middle or major envelope proteins System allows dissection of which immune response, targeted against which epitope, that is responsible for the immunopathology Cell-mediated Immunopathology: Human disease Evidence from mice model Transgenic mice expressing HbsAg Adoptive transfer of CTL specific for a HepB envelope protein results in acute hepatitis Direct cytotoxic effects of CTLs is limited to small numbers of hepatocytes (why? If all liver cells express the HBsAg) However, IFNg secreted by CTLs can attract other WBCs (phagocytes, PMNs) leading to necroinflammatory foci However, these cytokines are also involved in viral clearance; down regulates viral transcription Transgenic mice expressing large Hep B envelope protein (intracellular accumulation) Adoptive transfer of CTL specific for a HepB large envelope protein results in progessive inflammation and liver necrosis ER accumulation of L protein may be cytotoxic resulting in activation of liver macrophages (Kupffer cells) Antibody-mediated Immunopathology Ab-Ag complex formation during viremic infections Deposition of immune complex in glomerulus can initiate complement cascade and cause inflammation, scarring and eventual kidney failure Large complexes get trapped at the basement membrane Mesangial cells enlarge into the subepithelial (mesangial) space in an attempt to remove accumulating immune complexes Long term: glomerular capillary gets constricted foot processes of podocytes (glomerular endothelial cells) get fused; basement membrane gets leaky but filtering is blocked Result: Impaired glomerular function, kidney failure (no urine production) QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Antibody-mediated Immunopathology Ab-Ag complex formation during viremic infections Deposition of immune complex in glomerulus can initiate complement cascade and cause inflammation, scarring and Infectious Ab-Ag complex Can be demonstrated by reduction in infectious titer using antibodies against mouse IgG virus eventual kidney failure Y = anti-LCMV Y Y Y Y = anti-mouse IgG Y Y Treatment of sera From mice persistently infected w ith LCM V LCMV titer (log10 LD50 per 0.02 ml) Anti-mouse immun oglobulin <1.0 Controls Normal rabbit serum Anti-mouse albumin 3.7 3.5 Antibody-Mediated Immunopathology: Human Disease Dengue hemorrhagic fever Dengue virus, flavivirus transmittted by mosquitoes 4 serotypes (1-4) Disease is usually self-limiting but small percentage develop hemorrhagic fever and shock DHF/DSS occurs most commonly in children previously infected with a different serotype infants with primary infection but with maternal anti-dengue antibodies against another serotype Abs against one serotype can enhance infection by second serotype (Antibody-Dependent Enhancement) Y Y Enhancement is dependent on the titer of the Abs (Heterotypic anti-Dengue Ab) Fc Receptor monocyte Enhancement is dependent on Fc portion of Abs (Fab fragments from immune sera do not have Enhancing ability) Y ADE Y Total IgG F(ab)2 +++ - Enhancement is dependent on the titer of the Abs At high concentrations of virus-specific Ab (low Ab dilution), percent occupancy of antibody binding sites is sufficiently high to inhibit critical steps in the viral life cycle As antibody becomes more dilute, its inhibitory activity becomes attentuated, at some point below the neutralization end-point, antibody binding at subneutralizaing concentrations (High Ab dilution) enhances viral infectivity Neutralization end-point Enhanced attachment, free envelope spikes available to mediate fusion Y YY No free envelope spikes to mediate fusion Y Y Y Y (Heterotypic anti-Dengue Ab) Fc Receptor Fc Receptor monocyte monocyte (Heterotypic anti-Dengue Ab) Enhancement is dependent on the titer of the Abs Enhancement is dependent on Fc portion of Abs (Fab fragments from immune sera do not have Enhancing ability) Y ADE ADE Y Total IgG F(ab)2 +++ +++ YYY [+anti-F(ab)2] Fc How does ADE in secondary Dengue virus infection lead to DHF/DSS? Increased entry/replication in target cells (Monocytes/Macrophages) results in secretion of inflamatory cytokines (TNF-a, IFNg and IL-2) that also have vasoactive properties In 2º Dengue infection, pre-existing Ag-specific CD8 and CD4 T cells are activated, and also secrete similar vasoactive cytokines Resulting “cytokine” storm can capillary fragility (associated with hemorrhage) and permeability (associated with shock--loss of intravascular osmotic pressure) Anti-viral antibodies: the good, the bad, and the useless LCMV immunopathogenesis: A: Slowly replicating Armstrong strain Ab slows hematogenous spread of LCMV, and prevents robust CTL response; protects animals against CTL mediated choriomeningitis B: Intermediate replicating WE strain Virus infected meningeal cells become target for CTLs-resulting in choriomeningitis CTLs are made due to hematogenous spread to spleen and other lymphoid tissues Too high a level of viral replication in lymphoid tissue can lead to immune exhaustion: activation induced apoptosis of Ag-specific CTLs Ab did not slow spread of LCMV sufficiently to affect disease outcome C: High replicating strain Usually causes immune exhaustion (“high-dose paralysis”); Ab slows down virus enough for robust CTL response, which results in fatal choriomeningitis Virus-initiated autoimmunity: Molecular mimicry Immune response against a viral antigen cross-reacts with a host protein Cross-reactivity doesn’t necessarily result in autoimmune disease Experimental Allergic Encephalitis (EAE) Myelin Basic Protein immunization results in EAE (demyelination); “encephalitogenic” epitope confined to 10 amino-acid stretch in MBP HBV polymerase contains similar 10 aa stretch; immunization with this peptide can cause encephalitis in rabbits IMMUNOLOGICAL CROSS REACTIVITY DEMONSTRATED PROTEIN SEQUENCE Poliovirus VP2 Acetylcholine receptor STTKESRGTT TVIKESRGTK YES Papilloma virus E2 Insulin receptor SLHLESLKDS VYGLESLKDL YES Rabiesvirus glycoprotein Insulin receptor TKESLVIIS NKESLVISE YES HIV p24 IgG constant region GVETTTPS GVETTTPS YES Measles virus P3 Corticotrophin LECIRALK LECIRACK NO Virus-Induced Immunopathology Defensive Effects Destructive Effects ---CTL removal of infected cells ---CTL mediated destruction ---Ab neutralization ---ADE; Immune Complex Disease