* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Section 5.3 Physics and Quantum Mechanical Model
Elementary particle wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Atomic orbital wikipedia , lookup
Tight binding wikipedia , lookup
Particle in a box wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Electron configuration wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron scattering wikipedia , lookup
Double-slit experiment wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Matter wave wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
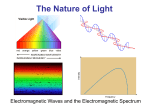
Section 5.3 Physics and Quantum Mechanical Model • The study of light led the development of the quantum mechanical model by Schrödinger’s. • Isaac Newton believed that light consisted of particles. • Scientists in the beginning of the 1900’s believed that light consisted of waves. Parts of a Wave • Amplitude: height from zero to the crest. • Wavelength (λ): the distance between the crests. • Frequency (υ): the number of wave cycles to pass a given point per unit of time. • Speed of light (c) equals the wavelength times the frequency c=λυ • The wavelength and frequency of light are inversely proportional to each other. c=λυ • According to the wave model, light consists of electromagnetic waves. • Electromagnetic radiation includes radio waves, microwaves, infrared waves, visible light ultraviolet waves, X-rays, and gamma rays. • All electromagnetic waves travel in a vacuum at a speed of 2.998 X 108m/s. Color Spectrum • Sunlight (white light) is a continuous range of wavelengths and frequencies. • A prism, or rain droplets in the case of a rainbow, can separate each frequency of light into a spectrum of colors. • Each color bends into the other in the order of red, orange, yellow, green, blue, and violet. – ROY G BIV • Which color in the visible spectrum has the longest wavelength? Practice Problem Calculating the Wavelength of Light Question • What is the frequency of radiation which has a wavelength of 7.00 X 10-5 cm? • In what region of the electromagnetic spectrum is this radiation? Answer • 4.29 X 1014 s-1 • Infrared Spectrum Atomic Spectra • When atoms absorb energy, electrons move into higher energy levels, and these electrons lose energy by emitting light when they return to lower energy levels. • Unlike white light, the light emitted by atoms consists of a mixture of only specific frequencies, each of a particular color. • Therefore, when light emitted by an element passes through a prism, it separates into discrete lines to give the atomic emission spectrum of that element. Atomic Emission Spectrum • Each discrete line in an emission spectrum corresponds to one exact frequency of light emitted by the atom. • Each emission spectrum is unique to that element. No two elements have the same spectrum. Niels Bohr • The Bohr Model, was on of the first models to explain the emission spectrum of hydrogen, and predicted specific values of these frequencies. • In the Bohr models the lone electron of hydrogen can have only certain specific energies. • The lowest energy is its ground state • In the ground state the principle quantum number (n) is 1. • Excitation of the electron by absorbing energy raises it from quantum from the ground state to an excited state with n = 2, 3, 4, and so forth. Ground State Emission of Light • When the electron looses energy and drops back to a lower energy level, energy (in the form of light) is released. • This happens in a single abrupt step called an electronic transition. Energy Equation • The light emitted by an electron moving from a higher to a lower energy level has a frequency directly proportional to the energy change of the electron. • Therefore, each each transition produces a line of specific frequency () in the spectrum Energy is related by: E = (h)() where; h = 6.626 X 10-34 J s plan = frequency 3 Groups of Lines Observed in the Emission Spectrum of Hydrogen Refer to Page 143 Figure 5.14 1. Lyman Series • • Ultraviolet Energy value of electrons from higher energy levels to n =1 2. Balmer Series • • Visible transitions from higher energy levels to n = 2 3. Paschen Series • • Infrared transitions from higher energy levels to n = 3 Questions 1. 2. 3. 4. 5. 6. What is the name of the series of visible lines in the hydrogen spectrum? Suppose an electron, in its ground state at n = 1, absorbs enough energy to jump to n = 2. What type of radiation will it emit when it returns to the ground state? If you observe a hydrogen gas discharge tube through a diffraction grating, could you see the line corresponding to this emission? Which series of lines could human detect with our eyes? Compare the energy of the Paschen and the Balmer series. What do you notice about the spacing of the energy levels from n = 1 to n = 7? Quantum Mechanics • Light – is it a wave or a particle? • Dual Wave-Particle Behavior of light • The particle aspect (as explained by Einstein) of light, could be describe as a quanta of energy. • Light quanta are called photons Photoelectric Effect • Metals eject electrons called photoelectrons when light shines on them. • Red light ( = 4.3 X 1014 s-1 to 4.6 X 1014 s-1), will not cause the ejection of photoelectrons from potassium metal. • Yellow light ( = 5.1 X 1014 s-1 to 4.3 X 1014 s-1) will. • Intensity does not matter. De Broglie • Reasoned that if light behaves as waves and particles, than particles of matter can also behave as waves. = h (m)() where; h = plank’s constant (6.626 X 10-34 J s) m = mass = frequency • Classical mechanics adequately describes the motions of bodies much larger than atoms, while quantum mechanics describes the motion of subatomic particles and atoms as waves. • Using the above equation, the wavelength of a moving electron (mass of an electron is 9.11 X 10-28g) and (moving at the speed of light) has a wavelength of about 2 X 10-10cm. (this is the size of a typical atom) Major Differences between Classical Mechanics and Quantum Mechanics 1. Classical mechanics adequately describes the motions of bodies much larger than the atoms they comprise. It appears that such a body gains or loses energy in any amount. 2. Quantum mechanics describes the motions of subatomic particles and atoms as waves. These particles gain or lose energy in packages called quanta. Heisenberg Uncertainty Principle • It is impossible to know exactly both the velocity and the position of a particle at the same time. • Schrödinger used the wavelike motion of matter and the uncertainty principle in his electron cloud model of an atom which lead to the concept of electron orbitals and configurations.