* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Quantum Mechanics and the Bohr Model - slater science
Ferromagnetism wikipedia , lookup
Particle in a box wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Canonical quantization wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Electron configuration wikipedia , lookup
Double-slit experiment wikipedia , lookup
Electron scattering wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Tight binding wikipedia , lookup
Hydrogen atom wikipedia , lookup
Matter wave wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
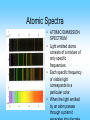
Physics and the Quantum Mechanical Model Objectives •Describe the relationship between the wavelength and frequency of light. •I.D. the source of atomic emission spectra. •Explain how the frequencies of emitted light are related to changes in electron energies. •Distinguish between quantum mechanics and classical mechanics. What are Electromagnetic Waves? • Electromagnetic waves are formed when an electric field (shown as red) couples with a magnetic field (shown as blue). • The magnetic and electric fields of an electromagnetic wave are perpendicular to each other and to the direction of the wave. Properties of Waves • On your paper draw a horizontal line approximately 5 inches long. • Using the line as the midpoint draw two waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. Electromagnetic Spectrum PRACTICE! Atomic Spectra • ATOMIC EMMISSION SPECTRUM • Light emitted atoms consists of a mixture of only specific frequencies. • Each specific frequency of visible light corresponds to a particular color. • When the light emitted by an atom passes through a prism it • The lowest possible energy of the electron is the ground state. (n=1) • Absorbing energy moves it to the excited state (n=2,3,4, 5, 6, 7) • E = h x • Planck's equation implies the higher the frequency of a radiation, the more energetic are its quanta. • E= quantum of Energy • = frequency • h= 6.626 x 10-34 J·s Bohr’s Hydrogen Model and Atomic Spectra • Suppose an electron in its ground state (n=1) absorbs enough energy to jump to level 2. What type of radiation will it emit when it returns to the ground state? • Which series of lines can be observed in the emission spectrum of hydrogen? • Compare the energy of the Paschen and Balmer series. • What do you notice about the spacing of the energy levels from n=1 to n=7? Science Trek • http://www.colorado.edu/physics/2000/q uantumzone/bohr.html Professor Edwards Melissa Sergio