* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 37 - USU Department of Physics
Quantum electrodynamics wikipedia , lookup
Anti-gravity wikipedia , lookup
Aristotelian physics wikipedia , lookup
Photon polarization wikipedia , lookup
Path integral formulation wikipedia , lookup
Quantum potential wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Electromagnetism wikipedia , lookup
Renormalization wikipedia , lookup
Bell's theorem wikipedia , lookup
Statistical mechanics wikipedia , lookup
Quantum mechanics wikipedia , lookup
Hydrogen atom wikipedia , lookup
Quantum vacuum thruster wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Relational approach to quantum physics wikipedia , lookup
Quantum tunnelling wikipedia , lookup
Fundamental interaction wikipedia , lookup
EPR paradox wikipedia , lookup
Nuclear physics wikipedia , lookup
History of quantum field theory wikipedia , lookup
Old quantum theory wikipedia , lookup
Classical mechanics wikipedia , lookup
History of subatomic physics wikipedia , lookup
History of physics wikipedia , lookup
Time in physics wikipedia , lookup
Condensed matter physics wikipedia , lookup
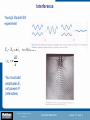
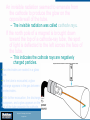
Physics of Technology PHYS 1800 Lecture 37 Introduction Quantum Mechanics in a Day Section 0 Lecture 1 Slide 1 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 1 PHYSICS OF TOF ECHNOLOGY - PHYS 1800 PHYSICS TECHNOLOGY ASSIGNMENT SHEET Spring 2009Spring Assignment Sheet 2009 Date Day Lecture Chapter Feb 16 M Presidents Day 17 Tu Angular Momentum (Virtual Monday) 18 W Review 19 H Test 2 20 F* Static Fluids, Pressure Feb 23 M Flotation 25 W Fluids in Motion 27 F* Temperature and Heat Mar 2 M First Law of Thermodynamics 4 W Heat flow and Greenhouse Effect 6 F* Climate Change Mar 9-13 M-F Spring Break Mar 16 M Heat Engines 18 W Power and Refrigeration 20 F* Electric Charge Mar 23 M Electric Fields and Electric Potential 25 W Review 26 H Test 3 27 F* Electric Circuits Mar 30 M Magnetic Force Review Apr 1 W Electromagnets 3 F Motors and Generators Apr 6 M Making Waves 8 W Sound Waves 10 F* E-M Waves, Light and Color Apr 13 M Mirrors and Reflections Introduction Section 0 Lecture 1 Slide 2 15 W Refraction and Lenses 17 F* Telescopes and Microscopes Apr 20 M Review 22 W Seeing Atoms 24 F The really BIG & the really small INTRODUCTION TO Modern Physics PHYX 2710 May 1 F Final Exam: 09:30-11:20am No Class 8 5-8 5-8 9 9 9 10 10 10 No Classes 11 11 12 12 13 9-12 13 14 9-12 14 15 15 16 17 17 17 1-17 18 (not on test) 21 (not on test) Homework Due - 6 7 8 - 9 10 11 No test week 12 Fall 2004 * = Homework Handout *Homework Handout Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 2 Interference Young’s Double Slit experiment X 1 X 2 n , n 0,1,2... D yn n d You must add amplitudes E, Introduction not powers P (intensities) Section 0 Lecture 1 Slide 3 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 3 Interference of Light Waves Is light a wave or a particle? – If it is a wave, it should exhibit interference effects: Recall that two waves can interfere constructively or destructively Introduction depending on their phase. Section 0 Lecture 1 Slide 4 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 4 Light from a single slit is split by passing through two slits, resulting in two light waves in phase with each other. The two waves will interfere constructively or destructively, depending on a difference in the path length. If the two waves travel equal distances to the screen, they interfere constructively and a bright spot or line is seen. Introduction Section 0 Lecture 1 Slide 5 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 5 If the distances traveled differ by half a wavelength, the two waves interfere destructively and a dark spot or line appears on the screen. If the distances traveled differ by a full wavelength, the two waves interfere constructively again resulting in another bright spot or line. The resulting interference pattern of alternating bright and dark lines is a fringe pattern. y path difference d x Introduction Section 0 Lecture 1 Slide 6 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 6 Similarly, interference can occur when light waves are reflected from the top and bottom surfaces of a soap film or oil slick. The difference in the path length of the two waves can produce an interference pattern. This is called thin-film interference. Introduction Section 0 Lecture 1 Slide 8 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 8 Different wavelengths of light interfere constructively or destructively as the thickness of the film varies. This results in the many different colors seen. Introduction Section 0 Lecture 1 Slide 9 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 9 The thin film may also be air between two glass plates. Each band represents a different thickness of film. Introduction Section 0 Lecture 1 Slide 10 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 10 Diffraction and Gratings The bright fringes in a double-slit interference pattern are not all equally bright. – They become less bright farther from the center. – They seem to fade in and out. This effect, called diffraction, is due to interference of light coming from different parts of the same slit or opening. Introduction Section 0 Lecture 1 Slide 11 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 11 Diffraction For constructive interference: Path Difference d sin( ) n , n 0,1,2... Introduction Section 0 Lecture 1 Slide 15 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 15 The diffraction pattern produced by a square opening has an array of bright spots. Looking at a star or distant street light through a window screen can produce a similar diffraction pattern. Introduction Section 0 Lecture 1 Slide 16 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 16 X-Ray Diffraction Introduction Section 0 Lecture 1 Slide 17 INTRODUCTION TO Modern Physics PHYX 2710 Braggs’s Law: nλ=2d sin(Θ) Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 17 Why study everyday phenomena? The same physical principles that govern our everyday experiences also govern the entire universe – A bicycle wheel, an atom, and a galaxy all operate according to laws for angular momentum. Introduction Section 0 Lecture 1 Slide 18 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 18 What are the major subfields in Physics? Classical Physics (pre 20th century) – – – – Mechanics → forces, motion Thermodynamics → heat, temperature Electricity and magnetism → charge, currents Optics → light, lenses, telescopes Modern Physics (20th century) – Atomic and nuclear → radioactivity, atomic power – Quantum mechanics } → basic structure matter – Particle physics Introduction Section 0 Lecture 1 Slide 19 – Condensed matter → solids and liquids, computers, lasers – Relativity, Cosmology → universe, life! INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 19 State of Physics cira 1895 Conservation Laws Electricity & Magnetism • Energy Statistical Mechanics • 3 Laws of Thermodynamics • Kinetic Theory Maxwell Equations (c 1880) • Gauss’ Law •Faraday’s Law •Ampere’s Law •No magnetic monopoles • Linear & Angular Momentum Mechanics (Gravity) Newton’s Laws (c 1640) 1-Law of inertia 2-F=ma 3-Equal and opposite reactions Introduction Section 0 Lecture 1 Slide 20 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 20 Limits of pre-Modern Physics Dimension Range of Applicability Range of Application Length 10-6 to 10+8 m Smoke particle (Brownian Motion) to the solar system Mass 10-9 to 10+31 kg Dust particles to solar mass Time 10+10 to 10+17 sec-1 10-3 to 10+9 sec Microwave to UV light Smallest timing increments (msec) to celestial motions (centuries) Lecture 1 -6 to 010+5 10Section m/s Small particles to celestial motion Velocity Introduction Slide 21 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 21 Then All Hell Broke Lose “Thirty Years That Shook Physics” • • • • • • • • • • • • • • • • • 1887 Michelson-Morley exp. debunks “ether” 1895 Rontgen discovers x rays 1897 Becquerel discovers radioactivity 1897 Thomson discovers the electron 1900 Planck proposes energy quantization 1905 Einstein proposes special relativity 1915 Einstein proposes general relativity 1911 Rutherford discovers the nucleus 1911 Braggs and von Laue use x rays to determine crystal structures 1911 Ones finds superconductors 1913 Bohr uses QM to explain hydrogen spectrum 1923 Compton demonstrates particle nature of light 1923 de Broglie proposes matter waves 1925 Davisson & Germer prove matter is wavelike 1925 Heisenberg states uncertainty principle 1926 Schrodinger develops wave equation Introduction Section 0 Lecture 1 Slide 22 1924-6 Boson and Fermion distributions developed • 1949 Murphy's Law stated INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 22 Current State of Physics cira 2009 Conservation Laws Statistical Mechanics • • • Electricity & Magnetism • • Physics of many particles • Fermions and Bosons • Partitioning of Energy • Thermodynamics • Time and Entropy Energy Linear & Angular Momentum Charge, Spin Lepton and Baryon Number Maxwell Equations (c 1880) Weinburg-Salom Model • QED • Unites E&M, Weak NF Mechanics (Gravity)…… Weak Nuclear Force General Relativity Space and time Radioactivity Standard Model Quantum Mechanics Introduction Section 0 •Schrodinger/Dirac Equation •Probabilistic approach Lecture 1 Slide 23 • QCD • Unites E&M, Strong NF, Weak NF Strong Nuclear Force INTRODUCTION TO Modern Physics PHYX 2710 Composition of subatomic particles Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 23 Limits of Current Modern Physics Dimension Range of Applicability Range of Application Length 10-18 to 10+26 m Quark size to the universe size Mass 10-31 to 10+40 kg Electrons to galactic clusters Time 10+3 to 10+22 sec-1 10-16 to 10+17 sec Radio to Gamma rays Sub-femtosecond spectroscopy to age of universe Velocity 10-8 to 10+8 m/s Introduction Section 0 Lecture 1 Slide 24 Sub-atomic particles to speed of light INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 24 Cathode rays, Electrons, and X-rays By the end of the nineteenth century, chemists were using the concept of atoms to explain their properties. Physicists were less convinced. – The discovery of cathode rays was the beginning of atomic physics. Two electrodes are sealed in a glass tube. As the tubeIntroduction is evacuated, a glow discharge Section 0 Lecture 1 Slide 25 appears in the gas between the electrodes. With further evacuation, the discharge disappears, and a glow appears on the end of the tube opposite the cathode. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 25 An invisible radiation seemed to emanate from the cathode to produce the glow on the opposite wall of the tube. – The invisible radiation was called cathode rays. If the north pole of a magnet is brought down toward the top of a cathode-ray tube, the spot of light is deflected to the left across the face of the tube. – This indicates the cathode rays are negatively charged particles. Two electrodes are sealed in a glass tube. As the tube is evacuated, a glow Introduction Section Lecture 1 discharge appears in the gas0 between the electrodes. Slide 26 With further evacuation, the discharge disappears, and a glow appears on the end of the tube opposite the cathode. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 26 J. J. Thomson used both electric fields and magnetic fields to deflect the beam. The combined effect allowed him to estimate the velocity of Introduction Section 0 Lecture 1 Slide 27 the particles. With the deflection produced by the magnetic field alone, this allowed him to estimate the mass of the particles. – We now call these particles electrons. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 27 You probably use cathode rays almost every day. The heart of most television sets is the cathode ray tube, or CRT. Introduction Section 0 Lecture 1 Slide 28 Do you know how a TV works? INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 28 The electrodes that produce and focus the electron beam are called the electron gun. – An electric current passes through the filament to heat the cathode to emit electrons. – Electrons are accelerated from the cathode to the anode by the high voltage. – Electrons passing through the hole in the anode make up the electron beam. After leaving the electron gun, the beam of electrons travel across the tube, producing a bright spot of light when it strikes the glass face of the tube. Magnets deflect the beam so that it strikes different points on the face of the tube at different times. Section 0 Lecture 1 Slide The beamIntroduction scans across the entire face of the tube in a fraction of a second, to form the picture. 29 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 29 Experiments on the “New”Particle” The Electron Introduction Section 0 Lecture 1 Slide 30 Robert Millikan measured the quantized charge on the electron INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 J.J. Thomson measured the charge-to-mass ratio e/m Quantum Mechanics Lecture 37 Slide 30 Davisson and Germer Experiment In 1927 Davisson and Germer For electrons: first demonstrated the diffraction patterns generated h 1.226 nm by electrons of 10eV passing 2mE eV through a Ni crystal. Introduction Section 0 Lecture 1 Slide 31 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 31 Electron Diffraction Energy Electron Diffraction: Electron and x-ray both exhibit diffraction from crystals. Introduction Section 0 Lecture 1 Slide 32 INTRODUCTION TO Modern Physics PHYX 2710 Braggs’s Law: nλ=2d sin(Θ) Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 32 Electron Interference Introduction Section 0 Lecture 1 Electron Single Slit Interference: Effects are clearly observed. However, as soon as “electron tracking” is instituted, the interference pattern disappears! Slide 33 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 33 Low Energy Electron Diffraction Typical LEED systems. (left) UHV surface analysis chamber. (right) LEED electron guns and grids. Introduction Section 0 Lecture 1 Slide 34 Low Energy Electron Diffraction is a standard tool in surface science. ~50 eV electrons with λ~1 Å are diffracted from surface atoms to determine atomic structure. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 34 LEED Images A SPA LEED image of silicon taken with 128 eV electrons. Introduction Section 0 Lecture 1 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Slide 35 The organic molecule PTCDI adsorbed on the 110 surface of Ag. Quantum Mechanics Lecture 37 Slide 35 Neutron Diffraction Section 0 Lecture 1 Slide 36 (left) TripleIntroduction axis neutron diffractometer at the NIST Neutron scattering facility. (right) Diffraction pattern from nuetrons. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 36 ...even Quantum Physics (matter waves)... Introduction Section 0 Lecture 1 Slide 37 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 37 Nobel Prizes Related to Wave-Particle Duality There have been 14 Nobel Prizes in physics awarded that have some direct relation to the wave-particle duality. Albert Einstein received the Nobel Prize for one of these, the photoelectric effect. Introduction Section 0 Lecture 1 Slide 38 Annotated list of winners. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 38 Pioneers in the Wave Theory of Particles Introduction Section 0 Lecture 1 Born Slide 39 Heisenburg Schrodinger INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 39 Particles as a Wave In 1924 de Broglie suggested that electrons may have wave properties. The wave length of an electron is proposed to be h/p where p is the momentum of the electron. De Broglie waves: • This expression is consistent with photon (E=pc) or p=h/ λ. h h p photon p particle mv • Because of the Planck constant, the λ of macroscopic object is not detectable. • Lower momentum particle is more wavelike. Introduction Section 0 Lecture 1 Slide h For electrons: 40 • Higher energy wave is more particle like. h 1.226 nm 2mE E INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 40 De Broglie Wave Problems Assume the mass of the jelly bean is 5 g and its velocity is 1 m/s. The total energy is just the kinetic energy E=KE=1/2 mv2 . 2 p Then E 1 102 J 6 1016 eV What is the de wave length of a jelly bean? 2m h p 6 1032 m ! And What is the de Broglie wave length of a Germer Introduction electron? For an electron all the electrostatic PE is converted to KE, eV 1/2 mv2 . First solve for p then for λ. Section 0 Lecture 1 First INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Then Physics of Technology—PHYS 1800 Spring 2009 Slide 41 2 p 8 1018 J 50 eV 2m h 6 1010 m ! E eV p Quantum Mechanics Lecture 37 Slide 41 Waves of what? The physical interpretation of de Broglie waves (the wave function or Schrodinger waves) is related to the probability of finding a moving particle at a particular location (x,y,z) and time t. • Because Ψ(x,y,z,t) is complex and can be positive or negative, it cannot be the probability directly. Born • | Ψ(x,y,z,t) |2 is the probabilityof finding the particle at location (x,y,z) at time t. Introduction Section 0 Lecture 1 Slide 42 • To be a probability function, it must obey a normalization condition 2stating it must be ( x, y, z) dV 1 somewhere: INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Schrodinger Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 42 The Schrödinger Equation 2 2 x, t x, t U ( x ) x , t i 2m x 2 t The Schrodinger Wave equation: U(x): Potential Energy P ( x, t ) 2 ( x, t ) :Complex wave function probability to find the particle at (x,t) ( x, t ) dx ( x) dx 1 normalization of the 2 2 wave function ( x, t ) ( x, t ) ( x, t ), , must be continuous , x Let Introduction Sectioni0t ( x, t ) ( x)e t Lecture 1 2 43d Slide x 2 2m dx 2 U ( x) x x 2 2 d x Time independent Schrödinger equation U ( x) x E x 2 2m dx INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 43 Matter is made up of atoms… The Atomic Theory, a cornerstone of modern science, was proposed by an early Greek thinker, Democritus (c.460 BC - c.370 BC). 2400 year later, Feynman deemed this the most important notion in science Introduction Section 0 Lecture 1 Slide 44 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 44 Trying to see atoms… Optical image STM Image (5 X mag) (3,000,000 X mag) SEM Image STM Image (300,000 X mag) (24,000,000 mag) Introduction Section 0 Lecture 1 Slide 45 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Magnified images of semiconductor chip. Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 45 Seeing atoms…finally!!! Introduction Section 0 Lecture 1 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Atomic scale images seen with scanning tunneling microscope STM developed in 1985 at IBM Measures extent of electron cloud Slide 46 Binnig and Roher’s original STM Quantum Mechanics Lecture 37 Slide 46 Examples of STM images… Pt (100) with vaccancies Si (111) 7x7 reconstructi on Introduction Section 0 Annealed decanethiol Lecture 1 Slide 47 film on Au(111) INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Si (111) with Quantum Mechanics terraces and Lecture 37 Slide 47 Examples of STM images (part 2)… Introduction Section 0 Lecture 1 Slide 48 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 48 Barrier Penetration (QuantumTunneling) V(x) V ( x) Vo V0 E 0 0 a ,0 xa Ψ is damped , otherwise Ψ oscillates x There is a certain probability T that the particle can tunnel through the barrier, a T Introduction e 0 2 k ( x ) dx 2 ka Lecture e 1 Section 0 where Slide 49 k 2mV ( x) E The thicker or higher the barrier, the less the tunneling probability approaches classical result INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 49 How an STM works… • An STM s a glorified phonograph needle • Tip motion uses piezioelectric crystals • Tunneling current results from overlap of electron wavefunction with conducting surface Introduction Section 0 Lecture 1 Slide 50 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 50 How an STM works (part 2)… Introduction Section 0 Lecture 1 Slide 51 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 51 Corralling electrons… Introduction Section 0 Lecture 1 Slide 52 STM used to make direct maps of the Quantum Mechanicsl probability distribution of the electron wave function of 2D state confined by “corrals” made of adsorbed atoms. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 52 Corralling electrons… Introduction Section 0 Lecture 1 Slide 53 STM used to make direct maps of the Quantum Mechanicsl probability distribution of the electron wave function of 2D state confined by “corrals” made of adsorbed atoms. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 53 Corralling electrons… Introduction Section 0 Lecture 1 Slide 54 STM used to make direct maps of the Quantum Mechanicsl probability distribution of the electron wave function of 2D state confined by “corrals” made of adsorbed atoms. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 54 Corralling electrons… Introduction Section 0 Lecture 1 Slide 55 STM used to make direct maps of the Quantum Mechanicsl probability distribution of the electron wave function of 2D state confined by “corrals” made of adsorbed atoms. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 55 Applications of knowledge on the atomic scale… Feynman: “Plenty of room at the bottom” – Inevitability of small – Interface of quantum mechanics with applications Introduction Section 0 Lecture 1 Slide 56 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 56 Moving atoms one layer at a time… Introduction Section 0 Lecture 1 Slide 57 Molecular Beam Epitaxy INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 57 Moving atoms one at a time… Introduction Section 0 Lecture 1 Slide 58 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 58 Moving atoms one at a time… Introduction Section 0 Lecture 1 Slide 59 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 59 Engineering Nanomachines Introduction Section 0 Lecture 1 Slide 60 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 60 Designer Molecules Introduction Section 0 Lecture 1 Slide 61 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 61 Moving many atoms Shen’s wor STM images of the H-terminated Si(100) surfaces Electron stimulated H/D-desorption from Si(100)-2x1 surface 200Åx200Å USU Nanolithography Lab Introduction 1x1 Dihydride Si H Section 0 Lecture 1 2x1 Monohydride 3x1 Monohydride + Dihydride Slide 62 TC Shen INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 62 Here at USU, TC Shen can make wires 1 atom wide! An atom is ~0.1 nm across. The moon is 4x108 m from the Earth (see front cover). How many atoms, in a 1 atom wide wire, would it take to reach the Moon? How much would this amount of Cu weigh? Introduction Section 0 Lecture 1 Slide 63 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 63 Atomic scale electronics Single electron transistor Chips ot the quantum scale Introduction Section 0 Lecture 1 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Slide 64 Transistors on a chip that switch with a single electron Quantum Mechanics Lecture 37 Slide 64 Quantum Computing Macro to Micro Micro to Nano Nano to Sub-nano Introduction Section 0 Lecture 1 Slide 65 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 65 Watching stuff happen… Introduction Chemistry time scales STM Femtosecond probes Section 0 Lecture 1 Slide 66 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 66 Femtosecond spectroscopy at USU… USU Femtosecond Spectroscopy Lab D. Mark Riffe Introduction Section 0 Lecture 1 Slide 67 Probing dynamics of hot electrons INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 67 What We Know About Atoms Nucleus protons+ neutrons 1-10 fermi (fm) e 1 fm=10-15 m Atomic number (Z)= number of protons p, n Atom Atoms are electrically neutral 0.1 nm= 1Å 1 Å=10-10 m Z= number of electrons Chemical properties are determined by electron configurations. Chemically active : single e (alkali) or vacancy (halogene) in the outer shell Introduction Section 0 Lecture 1 Slide 69 Chemically inert : completely filled outer shells Ion : atom lost or gained one or more electrons INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Valence electrons : electrons in the outer shell Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 69 Structural Models of the Atom Thomson “Plumb Pudding” Model Aristotle’s “Point” Model e 200 r Introduction Ze Section 0 Rutherford “Point Nucleus” Model Lecture 1 2 r 2a0 Slide 70 INTRODUCTION TO Modern Physics PHYX 2710 Bohr “Planetary” Model Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 QM “Probability” Model Quantum Mechanics Lecture 37 Slide 70 The Uncertainty Principle It can be shown that the minimum of the product of the two conjugate observables is 2 xp 2 and Et 2 Usually the uncertainty product is much greater than 2 If an excited energy of an atom has a lifetime τ, its energy cannot be known better than 2 Accuracy of the position measurements depends on wavelength. Smaller wavelength means more momentum. No observation will not disturb the subject. Introduction Section 0 Lecture 1 Slide 71 If a particle is confined in a space of length L, p 2 L Werner Heisenberg Uncertainty Principle 1927 The kinetic energy of the particle must be greater 2 2 Nobel Prize 1932 than a minimum, p Zero-point energy 2 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 2m 8mL Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 71 The Uncertainty Principle Changes in momentum Uncertainty in position Introduction Section 0 Lecture 1 Slide 72 determined by photon imparted by photon: probe wavelength: p h INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 p x h x Quantum Mechanics Lecture 37 Slide 72 Atomic Spectra Introduction Section 0 Lecture 1 Slide 73 Atomic Emission Spectrophotometer INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Atomic Emission Spectrum of Hydrogen Quantum Mechanics Lecture 37 Slide 73 The First Excited States Wave Functions of H-atom 1st excited state has three degenerated states 200 32 n=2, l=0, m=0 Zr Zr 2 a0 200 C200 2 e a0 n=2, l=1, m=0 210 C210 n=2, l=1, m=±1 211 C211 210 Zr Zr 2 a0 e cos a0 Zr Zr 2 a0 e sin e i a0 211 2 Introduction Section 0 r 2a0 2 pr 2 Lecture 1 210 r 2 2 200 r 2 2 Slide 74 2 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Bohr model: Quantum Mechanics r a0 6 4 r n2 a0 Z Lecture 37 Slide 74 Probability Distributions for Hydrogen Atom Introduction Section 0 Lecture 1 Slide 75 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 75 Periodic Table Introduction Section 0 Lecture 1 Slide 76 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 76 Formation of Bands in Solids Introduction Section 0 Lecture 1 Slide 77 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 77 An integrated circuit consists of several transistors, diodes, resistors, and electrical connections all built into a single tiny chip of semiconductor material, usually silicon. – This allows the production of circuits much smaller than circuits made from individual transistors or vacuum tubes. – A computer that would fill a large room can now be reduced to the size of a hand-held calculator. The starting point in producing integrated circuits is a polished wafer of singlecrystal silicon. Several identical Introduction Section 0 circuits are usually imprinted on a single silicon wafer. Lecture 1 Slide 78 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 78 – The wafer is cut into individual chips, each containing a miniature circuit. – The final steps involve making electrical connections to the chip, packaging the chip in a sealed plastic enclosure, and testing the resulting circuit. Introduction Section 0 Lecture 1 Slide 79 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 79 – Rows of packaged microchips are used on a single circuit board of a computer. – Competition to produce ever smaller and faster circuitry continues to push the technology forward. Research in the condensed-matter physics of semiconducting elements and compounds has become one of the most active areas in modern physics. Introduction Section 0 The revolution in electronics technology is still proceeding. Lecture 1 Slide 80 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 80 Superconductors and Other New Materials Superconductivity is a phenomenon in which the resistance to the flow of electric current completely disappears. The resistance of many metals drops abruptly to zero at the critical temperature Tc. Introduction Section 0 once Lecture 1 An electric current, started, would flow indefinitely with no source of power. Slide 81 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 81 The Meissner Effect One striking property of a superconductor is that it will completely exclude magnetic field lines produced by an external magnet or electrical current. A magnet brought near a superconducting material will be repelled. Section 0 Lecture 1 Slide 82 A smallIntroduction magnet can levitate above a superconducting disk cooled with liquid nitrogen. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 82 Physics of Technology Next Recitation: Math and Problem Solving Review Tuesday 1:30-2:45 ESLC 53 Review Appendices A,B,C Next Class: Introduction Section 0 Lecture 1 Wed Slide 83 10:30-11:20 BUS 318 room. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 83 Inconsistencies in Physics cira 2009 Statistical Mechanics • Fermions, Bosons and Anyons •Bose-Einstein Condensates •Superconductivity •Stellar Evolution • SM of Black Holes • Time and Entropy Conservation Laws • • • Electricity & Magnetism • Maxwell Equations (c1880) Energy Linear & Angular Momentum Charge, Spin Lepton and Baryon Number Heavy Fermions and HTSC Chaotic and complex systems Gravity……………… • • • • • General Relativity Space and time Inconsistent with QM Search for dark matter Fixed gravitational constant? Weak Nuclear Force • Radioactivity • CPT violations? GUT’s and TOE’s Quantum Mechanics Section 0 Lecture 1 Slide 84 • Existence ofIntroduction atoms Strong Nuclear Force •Schrodinger/Dirac Equation •Composition of subatomic particles • Sub-atomic particles •Matter/anitmatter imbalance • Probabilistic approach •Decay ratios and particle masses •Teleportation INTRODUCTION TO Modern Physics PHYX 2710 •Search for Higgs Bosons Fall 2004 •Entwined states •Nature of strong hadron force •Sub-Planck length physics •Proton decay Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics • Combining Standard model and Gravity • String Theory Lecture 37 Slide 84 The forces in the universe have also been grouped into a few fundamental forces: – The primary force responsible for binding the quarks in neutrons, protons, and other baryons and mesons is the strong nuclear interaction. – The weak nuclear force is involved in the interactions of leptons, such as beta decay. – The electric force and magnetic force have been combined into the electromagnetic force. Introduction Section 0 Lecture 1 Slide 85 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 85 – The standard model has now unified the electromagnetic force with the weak nuclear force to form the electroweak force. – A Grand unified theory (GUT) which will unify the strong force with the electroweak force is much sought after. – This leaves only the gravitational force. – A Theory of everything (TOE) may someday unify all forces, including gravity. Introduction Section 0 Lecture 1 Slide 86 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 86 Have you ever seen an atom? Why do we think atoms exist? Introduction Section 0 Do you believe in atoms? Lecture 1 Slide 87 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 87 Radioactivity and the Discovery of the When Becquerel placed aNucleus piece of phosphorescent material on a covered photographic plate, the developed plate showed a silhouette of the sample. Radiation apparently was passing from these materials to expose the film. Introduction Section 0 Lecture 1 Slide 88 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 88 The basic building blocks of the nucleus are the proton and the neutron. – Their masses are nearly equal. – The proton has a charge of +1e while the neutron is electrically neutral. This explains both the charge and the mass of the nucleus. – An alpha particle with charge +2e and mass 4 x mass of the proton is composed of two protons and two neutrons. – A nitrogen nucleus with a mass 14 times the mass of a hydrogen nucleus and a charge 7 times that of hydrogen is composed of seven protons and seven neutrons. Introduction Section 0 Lecture 1 Slide 89 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 89 Introduction Section 0 Lecture 1 Slide 90 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 90 Radioactive Decay Becquerel discovered natural radioactivity in 1896. By 1910, Rutherford and others demonstrated that one element was actually being changed into another during radioactive decay. The nucleus of the atom itself is modified when a decay occurs. – For example, Marie and Pierre Curie isolated the highly radioactive element radium which emitted primarily alpha particles. Introduction Section 0 Lecture 1 Slide 91 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 91 Nuclear Reactions and Nuclear Fission In addition to spontaneous radioactive decays, changes in the nucleus may be produced experimentally through nuclear reactions. Introduction Section 0 Lecture 1 Slide 95 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 95 Nuclear Reactors Fermi’s strategy to achieve a chain reaction with natural or slightly enriched uranium: – Slow the neutrons down between fission reactions using a material called a moderator. – Control rods are used to absorb the neutrons to slow the reaction as desired. Fermi’s “pile” was the first human-produced nuclear reactor. Introduction Section 0asLecture Graphite blocks served the moderator. Control rods were cadmium, but today’s reactors use boron. 1 Slide 96 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 96 Modern Power Reactors Most reactors today use ordinary (light) water as the moderator. This requires enrichment of the fuel to 3% U-235. The advantage is that the water can also be used as a coolant. Introduction Section 0 Lecture 1 Slide 97 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 97 A hydrogen bomb involves nuclear fusion rather than fission. – Nuclear fusion is another kind of nuclear reaction that also releases large quantities of energy. – Fusion is the energy source of the sun and other stars as well as of thermonuclear bombs. – In a sense, it is the opposite of fission: very small nuclei such as hydrogen, helium, and lithium combine to form larger nuclei. – As long as the mass of the reaction products is less than the mass of the original isotopes, energy is released. One possible reaction is the combination of two isotopes of hydrogen, deuterium and tritium, toIntroduction form helium-4 plus Section 0 Lecture a neutron: 1 Slide 101 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 101 Can we generate power from controlled fusion? Producing fusion reactions for a commercial power source has not yet been accomplished. – Confining the fuel at very high temperatures in a very small space presents extreme difficulties. Experimental reactors such as the Tokamak Fusion Test Reactor at Princeton, NJ, have generated energy from fusion, but they have not reached the break-even point, where as much energy Section 0 to Lecture is releasedIntroduction as is required initiate the reaction. Research on this problem may someday reach that goal. 1 Slide 103 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 103 Quarks and Other Elementary Particles Atoms were once thought to be the basic building blocks of all matter. We now know atoms consist of electrons, protons, and neutrons. Neutrons and protons also have a substructure of quarks. Introduction Section 0 Lecture 1 Slide 104 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 104 Where will this all end? What are quarks and why do we believe they exist? Will we someday discover that quarks also have a substructure? Recent advances in high-energy physics have produced the standard model. Particle accelerators like CERN in Europe and Fermilab in the U.S. are used to bombard targets with fast-moving particles. Particle detectors are used to study what emerges from these Introduction Section 0 Lecture 1 collisions. For example, particle tracks in a bubble chamber provide information on the new particles produced in collisions or decays. Slide 105 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 105 ... to the largest. How was the universe formed? How is it changing? Introduction Section 0 Lecture 1 Slide 106 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 106 The Michelson-Morley Experiment Michelson and Morley used an interferometer to detect small differences in the velocity of light or in the distance that the light traveled. Light waves traveling along the two perpendicular arms interfere to form a Introduction Section 0 Lecture 1 pattern of light and dark fringes. Slide 107 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 107 The Michelson-Morley Experiment At some time during the year the earth should be moving relative to the ether. No fringe shift was observed; the experiment failed to detect any motion of the earth relative to the ether. This “failure” was a very important Introduction Section 0result! Lecture 1 Slide 108 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 108 Einstein’s Postulates of Special Relativity Einstein’s solution to the dilemma of the ether and the speed of light was both simple and radical. – Postulate 1: The laws of physics are the same in any inertial frame of reference. – Postulate 2: The speed of light in a vacuum is the same in any inertial frame of reference, regardless of the relative motion of the source and observer. The firstIntroduction is just a reaffirmation of the principle of Section 0 Lecture 1 Slide 109 relativity stated earlier. The second is much more radical: light does not behave like most waves or moving objects. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 109 Newton’s Laws and Mass-Energy Equivalence Accepting Einstein’s postulates requires some major changes in how we think about space and time. For example, does Newton’s second law of motion still apply when objects are moving at large velocities? F = ma = p / t Introduction Section 0 Lecture 1 Slide 110 In order to maintain conservation of momentum, Einstein redefined momentum as p = mv INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 110 General Relativity What happens if our frame of reference is accelerating? Imagine that we are in a moving elevator, for example. If the elevator is moving with constant velocity, no experiment that we can do inside the elevator could establish whether or not we are moving. Introduction Section 0 Lecture 1 Slide 111 If the elevator is accelerating, a bathroom scale would register a greater weight. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 111 Similarly, space is curved near a very strong gravitational field. – This represents how things might be pulled into the center of the field. – Since light rays are bent by strong gravitational fields, they can be pulled into the center of the field as well as particles having some mass. Introduction Section 0 Lecture 1 Slide 112 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 112 This figure is a two-dimensional representation of a black hole. – Black holes are thought to be very massive collapsed stars, which generate an extremely strong gravitational field. – Space is very curved in their vicinity. Introduction Section 0 Lecture 1 Slide 113 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 113 Einstein’s theories of special and general relativity have had an enormous impact on our concepts of space and time. Special relativity deals with reference frames that, although moving at speeds near the speed of light, are still inertial (nonaccelerating). General relativity deals with accelerated reference frames. The predictions of these theories have been well confirmed. – For example, the energy released in nuclear reactions is a result of mass-energy equivalence. Section 0 Lecture 1 Slide 114 – Also, Introduction astronomical observations of the bending of starlight is evidence of the principle of equivalence between gravity and acceleration. These ideas excite the imagination and are still very active areas of research. INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 114 Cosmology and the Beginning of Time We have now looked into the extremely small: – Quarks make up protons and neutrons, which form the nucleus. – Atoms consist of the nucleus and the surrounding electrons. – Atoms make up molecules and the ordinary matter of our world. What about the very large? – The earth is part of the solar system which includes the sun. – The sun is just one star in our galaxy, which is just one galaxy in our Local Group of galaxies. – These groups of galaxies make up larger groups, and ultimately, the universe. What can our knowledge of atoms, nuclei, and quarks tell us about the universe? Introduction Section 0 Lecture 1 Slide 115 INTRODUCTION TO Modern Physics PHYX 2710 Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 115 Hurtzsprung-Russell (H-R) Diagram 100R 1000R Betelgeuse Polaris 1R Antares Arcturus Sirius A 0.01R Sun Sirius B Introduction Section 0 Lecture 1 Slide 116 LM4 Physics M PHYX 2710 INTRODUCTION T TOeModern Fall 2004 Physics of Technology—PHYS 1800 Spring 2009 Quantum Mechanics Lecture 37 Slide 116